Physical characterization of MxiH and PrgI, the needle component of the type III secretion apparatus from Shigella and Salmonella
- PMID: 16501225
- PMCID: PMC2249775
- DOI: 10.1110/ps.051733506
Physical characterization of MxiH and PrgI, the needle component of the type III secretion apparatus from Shigella and Salmonella
Abstract
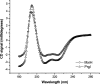
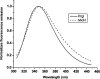
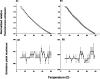
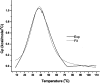
Shigella and Salmonella use similar type III secretion systems for delivering effector proteins into host cells. This secretion system consists of a base anchored in both bacterial membranes and an extracellular "needle" that forms a rod-like structure exposed on the pathogen surface. The needle is composed of multiple subunits of a single protein and makes direct contact with host cells to facilitate protein delivery. The proteins that make up the needle of Shigella and Salmonella are MxiH and PrgI, respectively. These proteins are attractive vaccine candidates because of their essential role in virulence and surface exposure. We therefore isolated, purified, and characterized the monomeric forms of MxiH and PrgI. Their far-UV circular dichroism spectra show structural similarities with hints of subtle differences in their secondary structure. Both proteins are highly helical and thermally unstable, with PrgI having a midpoint of thermal unfolding (Tm) near 37 degrees C and MxiH having a value near 42 degrees C. The two proteins also have comparable intrinsic stabilities as measured by chemically induced (urea) unfolding. MxiH, however, with a free energy of unfolding (DeltaG degrees 0,un) of 1.6 kcal/mol, is slightly more stable than PrgI (1.2 kcal/mol). The relatively low m-values obtained for the urea-induced unfolding of the proteins suggest that they undergo only a small change in solvent-accessible surface area. This argues that when MxiH and PrgI are incorporated into the needle complex, they obtain a more stable structural state through the introduction of protein-protein interactions.
Figures






References
-
- Blocker, A., Jouihri, N., Larquet, E., Gounon, P., Ebel, F., Parsot, C., Sansonetti, P., and Allaoui, A. 2001. Structure and composition of the Shigella flexneri “needle complex,” a part of its type III secretion. Mol. Microbiol. 39: 652–663. - PubMed
-
- Cordes, F.S., Komoriya, K., Larquet, E., Yang, S., Egelman, E.H., Blocker, A., and Lea, S.M. 2003. Helical structure of the needle of the type III secretion system of Shigella flexneri. J. Biol. Chem. 278: 17103–17107. - PubMed
-
- Cordes, F.S., Daniell, S., Kenjale, R., Saurya, S., Picking, W.L., Picking, W.D., Booy, F., Lea, S.M., and Blocker, A. 2005. Helical packing of needles from functionally altered Shigella type III secretions systems. J. Mol. Biol. 354: 206–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

