Viral nanoparticles as tools for intravital vascular imaging
- PMID: 16501571
- PMCID: PMC2536493
- DOI: 10.1038/nm1368
Viral nanoparticles as tools for intravital vascular imaging
Abstract
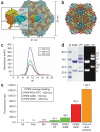
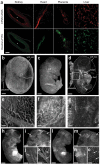
A significant impediment to the widespread use of noninvasive in vivo vascular imaging techniques is the current lack of suitable intravital imaging probes. We describe here a new strategy to use viral nanoparticles as a platform for the multivalent display of fluorescent dyes to image tissues deep inside living organisms. The bioavailable cowpea mosaic virus (CPMV) can be fluorescently labeled to high densities with no measurable quenching, resulting in exceptionally bright particles with in vivo dispersion properties that allow high-resolution intravital imaging of vascular endothelium for periods of at least 72 h. We show that CPMV nanoparticles can be used to visualize the vasculature and blood flow in living mouse and chick embryos to a depth of up to 500 microm. Furthermore, we show that the intravital visualization of human fibrosarcoma-mediated tumor angiogenesis using fluorescent CPMV provides a means to identify arterial and venous vessels and to monitor the neovascularization of the tumor microenvironment.
Figures



Similar articles
-
Intravital imaging of embryonic and tumor neovasculature using viral nanoparticles.Nat Protoc. 2010 Aug;5(8):1406-17. doi: 10.1038/nprot.2010.103. Epub 2010 Jul 8. Nat Protoc. 2010. PMID: 20671724 Free PMC article.
-
Evaluation of nanoparticle uptake in tumors in real time using intravital imaging.J Vis Exp. 2011 Jun 21;(52):2808. doi: 10.3791/2808. J Vis Exp. 2011. PMID: 21730939 Free PMC article.
-
Cowpea mosaic virus nanoparticles for cancer imaging and therapy.Adv Drug Deliv Rev. 2019 May;145:130-144. doi: 10.1016/j.addr.2019.04.005. Epub 2019 Apr 17. Adv Drug Deliv Rev. 2019. PMID: 31004625 Review.
-
Endothelial targeting of cowpea mosaic virus (CPMV) via surface vimentin.PLoS Pathog. 2009 May;5(5):e1000417. doi: 10.1371/journal.ppat.1000417. Epub 2009 May 1. PLoS Pathog. 2009. PMID: 19412526 Free PMC article.
-
Intravital fluorescence videomicroscopy to study tumor angiogenesis and microcirculation.Neoplasia. 2000 Jan-Apr;2(1-2):53-61. doi: 10.1038/sj.neo.7900062. Neoplasia. 2000. PMID: 10933068 Free PMC article. Review.
Cited by
-
Novel roles for well-known players: from tobacco mosaic virus pests to enzymatically active assemblies.Beilstein J Nanotechnol. 2016 Apr 25;7:613-29. doi: 10.3762/bjnano.7.54. eCollection 2016. Beilstein J Nanotechnol. 2016. PMID: 27335751 Free PMC article. Review.
-
Interaction of cowpea mosaic virus nanoparticles with surface vimentin and inflammatory cells in atherosclerotic lesions.Nanomedicine (Lond). 2012 Jun;7(6):877-88. doi: 10.2217/nnm.11.185. Epub 2012 Mar 6. Nanomedicine (Lond). 2012. PMID: 22394183 Free PMC article.
-
Recent Trends of the Bio-Inspired Nanoparticles in Cancer Theranostics.Front Pharmacol. 2019 Oct 25;10:1264. doi: 10.3389/fphar.2019.01264. eCollection 2019. Front Pharmacol. 2019. PMID: 31708785 Free PMC article. Review.
-
Sensitivity of cancer cells to truncated diphtheria toxin.PLoS One. 2010 May 5;5(5):e10498. doi: 10.1371/journal.pone.0010498. PLoS One. 2010. PMID: 20463924 Free PMC article.
-
Transferrin-mediated targeting of bacteriophage HK97 nanoparticles into tumor cells.Nanomedicine (Lond). 2011 Jan;6(1):55-68. doi: 10.2217/nnm.10.99. Nanomedicine (Lond). 2011. PMID: 21182418 Free PMC article.
References
-
- McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat. Med. 2003;9:713–725. - PubMed
-
- Yang CS, et al. Nanoparticle-based in vivo investigation on blood-brain barrier permeability following ischemia and reperfusion. Anal. Chem. 2004;76:4465–4471. - PubMed
-
- Josephson L, Kircher MF, Mahmood U, Tang Y, Weissleder R. Near-infrared fluorescent nanoparticles as combined MR/optical imaging probes. Bioconjug. Chem. 2002;13:554–560. - PubMed
-
- Lee PJ, Peyman GA. Visualization of the retinal and choroidal microvasculature by fluorescent liposomes. Methods Enzymol. 2003;373:214–233. - PubMed
-
- Rizzo V, Steinfeld R, Kyriakides C, DeFouw DO. The microvascular unit of the 6-day chick chorioallantoic membrane: a fluorescent confocal microscopic and ultrastructural morphometric analysis of endothelial permselectivity. Microvasc. Res. 1993;46:320–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

