Inhibition of fatty acid amide hydrolase produces analgesia by multiple mechanisms
- PMID: 16501580
- PMCID: PMC1617043
- DOI: 10.1038/sj.bjp.0706699
Inhibition of fatty acid amide hydrolase produces analgesia by multiple mechanisms
Erratum in
- Br J Pharmacol. 2006 May;148(1):114
Abstract
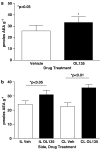
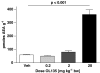
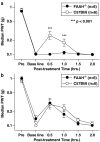
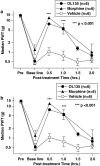
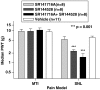
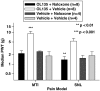
1 The reversible fatty acid amide hydrolase (FAAH) inhibitor OL135 reverses mechanical allodynia in the spinal nerve ligation (SNL) and mild thermal injury (MTI) models in the rat. The purpose of this study was to investigate the role of the cannabinoid and opioid systems in mediating this analgesic effect. 2 Elevated brain concentrations of anandamide (350 pmol g(-1) of tissue vs 60 pmol g(-1) in vehicle-treated controls) were found in brains of rats given OL135 (20 mg kg(-1)) i.p. 15 min prior to 20 mg kg(-1) i.p. anandamide. 3 Predosing rats with OL135 (2-60 mg kg(-1) i.p.) 30 min before administration of an irreversible FAAH inhibitor (URB597: 0.3 mg kg(-1) intracardiac) was found to protect brain FAAH from irreversible inactivation. The level of enzyme protection was correlated with the OL135 concentrations in the same brains. 4 OL135 (100 mg kg(-1) i.p.) reduced by 50% of the maximum possible efficacy (MPE) mechanical allodynia induced by MTI in FAAH(+/+)mice (von Frey filament measurement) 30 min after dosing, but was without effect in FAAH(-/-) mice. 5 OL135 given i.p. resulted in a dose-responsive reversal of mechanical allodynia in both MTI and SNL models in the rat with an ED(50) between 6 and 9 mg kg(-1). The plasma concentration at the ED(50) in both models was 0.7 microM (240 ng ml(-1)). 6 In the rat SNL model, coadministration of the selective CB(2) receptor antagonist SR144528 (5 mg kg(-1) i.p.), with 20 mg kg(-1) OL135 blocked the OL135-induced reversal of mechanical allodynia, but the selective CB(1) antagonist SR141716A (5 mg kg(-1) i.p.) was without effect. 7 In the rat MTI model neither SR141716A or SR144528 (both at 5 mg kg(-1) i.p.), or a combination of both antagonists coadministered with OL135 (20 mg kg(-1)) blocked reversal of mechanical allodynia assessed 30 min after dosing. 8 In both the MTI model and SNL models in rats, naloxone (1 mg kg(-1), i.p. 30 min after OL135) reversed the analgesia (to 15% of control levels in the MTI model, to zero in the SNL) produced by OL135.
Figures


References
-
- AHLUWALIA J., URBAN L., BEVAN S., NAGY I. Anandamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. Eur. J. Neurosci. 2003;17:2611–2618. - PubMed
-
- AHLUWALIA J., URBAN L., CAPOGNA M., BEVAN S., NAGY I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. - PubMed
-
- BISOGNO T., DE PETROCELLIS L., DI MARZO V. Fatty acid amide hydrolase, an enzyme with many bioactive substrates. Possible therapeutic implications. Curr. Pharm. Des. 2002;8:533–547. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

