Expression of Ca2+-dependent synaptotagmin isoforms in mouse and rat parotid acinar cells
- PMID: 16502487
- PMCID: PMC2687583
- DOI: 10.3349/ymj.2006.47.1.70
Expression of Ca2+-dependent synaptotagmin isoforms in mouse and rat parotid acinar cells
Abstract
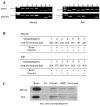
Synaptotagmin is a Ca2+ sensing protein, which triggers a fusion of synaptic vesicles in neuronal transmission. Little is known regarding the expression of Ca2+-dependent synaptotagmin isoforms and their contribution to the release of secretory vesicles in mouse and rat parotid acinar cells. We investigated a type of Ca2+-dependent synaptotagmin and Ca2+ signaling in both rat and mouse parotid acinar cells using RT-PCR, microfluorometry, and amylase assay. Mouse parotid acinar cells exhibited much more sensitive amylase release in response to muscarinic stimulation than did rat parotid acinar cells. However, transient [Ca2+]i increases and Ca2+ influx in response to muscarinic stimulation in both cells were identical, suggesting that the expression or activity of the Ca2+ sensing proteins is different. Seven Ca2+-dependent synaptotagmins, from 1 to 7, were expressed in the mouse parotid acinar cells. However, in the rat parotid acinar cells, only synaptotagmins 1, 3, 4 and 7 were expressed. These results indicate that the expression of Ca2+-dependent synaptotagmins may contribute to the release of secretory vesicles in parotid acinar cells.
Figures

Similar articles
-
Extracellular ATP increases free cytosolic calcium in rat parotid acinar cells. Differences from phospholipase C-linked receptor agonists.Biochem J. 1988 Oct 1;255(1):291-300. Biochem J. 1988. PMID: 2848507 Free PMC article.
-
A primary culture of parotid acinar cells retaining capacity for agonists-induced amylase secretion and generation of new secretory granules.Cell Tissue Res. 2005 Jun;320(3):455-64. doi: 10.1007/s00441-005-1076-x. Epub 2005 Apr 22. Cell Tissue Res. 2005. PMID: 15846515
-
Intracellular calcium signalling in rat parotid acinar cells that lack secretory vesicles.Biochem J. 1998 Mar 1;330 ( Pt 2)(Pt 2):847-52. doi: 10.1042/bj3300847. Biochem J. 1998. PMID: 9480900 Free PMC article.
-
Divergence and convergence in regulated exocytosis: the characteristics of cAMP-dependent enzyme secretion of parotid salivary acinar cells.Cell Signal. 1998 Jun;10(6):371-5. doi: 10.1016/s0898-6568(97)00178-2. Cell Signal. 1998. PMID: 9720759 Review.
-
Synaptotagmin IV acts as a multi-functional regulator of Ca2+-dependent exocytosis.Neurochem Res. 2011 Jul;36(7):1222-7. doi: 10.1007/s11064-010-0352-7. Epub 2010 Dec 10. Neurochem Res. 2011. PMID: 21153436 Review.
Cited by
-
The high-affinity calcium sensor synaptotagmin-7 serves multiple roles in regulated exocytosis.J Gen Physiol. 2018 Jun 4;150(6):783-807. doi: 10.1085/jgp.201711944. Epub 2018 May 24. J Gen Physiol. 2018. PMID: 29794152 Free PMC article. Review.
-
Expression, localization, and functional role for synaptotagmins in pancreatic acinar cells.Am J Physiol Gastrointest Liver Physiol. 2011 Aug;301(2):G306-16. doi: 10.1152/ajpgi.00108.2011. Epub 2011 Jun 2. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21636530 Free PMC article.
-
Ca²⁺-regulated secretory granule exocytosis in pancreatic and parotid acinar cells.Cell Calcium. 2014 Jun;55(6):369-75. doi: 10.1016/j.ceca.2014.03.003. Epub 2014 Mar 28. Cell Calcium. 2014. PMID: 24742357 Free PMC article. Review.
-
Molecular Regulatory Mechanism of Exocytosis in the Salivary Glands.Int J Mol Sci. 2018 Oct 17;19(10):3208. doi: 10.3390/ijms19103208. Int J Mol Sci. 2018. PMID: 30336591 Free PMC article. Review.
References
-
- Quissell DO. Stimulus-exocytosis coupling mechanism in salivary gland cells. In: Dobrosielski-Vergona K, editor. Biology of the Salivary Glands. Florida: CRC Press; 1993. pp. 181–200.
-
- Takuma T, Ichida T. Catalytic subunit of protein kinase A induces amylase release from streptolysin O-permeabilized parotid acini. J Biol Chem. 1994;269:22124–22128. - PubMed
-
- Turner RJ, Sugiya H. Understanding salivary fluid and protein secretion. Oral Dis. 2002;8:3–11. - PubMed
-
- Takuma T, Ichida T. Does cyclic AMP mobilize calcium for amylase secretion from rat parotid cells? Biochim Biophys Acta. 1986;887:113–117. - PubMed
-
- Butcher FR, Putney JW. Regulation of parotid gland function by cyclic nucleotides and calcium. Adv Cyclic Nucleotide Res. 1980;13:215–249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

