Depression of presynaptic excitation by the activation of vanilloid receptor 1 in the rat spinal dorsal horn revealed by optical imaging
- PMID: 16503963
- PMCID: PMC1434724
- DOI: 10.1186/1744-8069-2-8
Depression of presynaptic excitation by the activation of vanilloid receptor 1 in the rat spinal dorsal horn revealed by optical imaging
Abstract
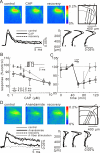
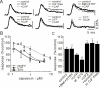
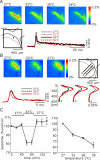
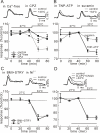
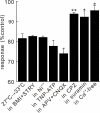
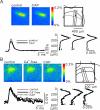
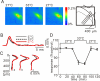
In this study, we show that capsaicin (CAP) depresses primary afferent fiber terminal excitability by acting on vanilloid receptor 1 (TRPV1 channels) of primary afferent fibers in adenosine 5'-triphosphate (ATP)- and temperature-dependent manner using two optical imaging methods. First, transverse slices of spinal cord were stained with a voltage-sensitive dye and the net excitation in the spinal dorsal horn was recorded. Prolonged treatment (>20 min) with the TRPV1 channel agonist, CAP, resulted in a long-lasting inhibition of the net excitation evoked by single-pulse stimulation of C fiber-activating strength. A shorter application of CAP inhibited the excitation in a concentration-dependent manner and the inhibition was reversed within several minutes. This inhibition was Ca(++)-dependent, was antagonized by the TRPV1 channel antagonist, capsazepine (CPZ), and the P(2)X and P(2)Y antagonist, suramin, and was facilitated by the P(2)Y agonist, uridine 5'-triphosphate (UTP). The inhibition of excitation was unaffected by bicuculline and strychnine, antagonists of GABA(A) and glycine receptors, respectively. Raising the perfusate temperature to 39 degrees C from 27 degrees C inhibited the excitation (-3%/ degrees C). This depressant effect was antagonized by CPZ and suramin, but not by the P(2)X antagonist, 2', 3'-O-(2,4,6-trinitrophenyl) adenosine 5'-triphosphate (TNP-ATP). Second, in order to record the presynaptic excitation exclusively, we stained the primary afferent fibers anterogradely from the dorsal root. CAP application and a temperature increase from 27 degrees C to 33 degrees C depressed the presynaptic excitation, and CPZ antagonized these effects. Thus, this study showed that presynaptic excitability is modulated by CAP, temperature, and ATP under physiological conditions, and explains the reported central actions of CAP. These results may have clinical importance, especially for the control of pain.
Figures
Similar articles
-
GABA(A) receptor facilitation of neurokinin release from primary afferent terminals in the rat spinal cord.Neuroscience. 2005;130(4):1013-27. doi: 10.1016/j.neuroscience.2004.10.019. Neuroscience. 2005. PMID: 15652997
-
Transient receptor potential vanilloid type 1 receptor regulates glutamatergic synaptic inputs to the spinothalamic tract neurons of the spinal cord deep dorsal horn.Neuroscience. 2009 May 5;160(2):508-16. doi: 10.1016/j.neuroscience.2009.02.019. Epub 2009 Feb 21. Neuroscience. 2009. PMID: 19236908
-
Effect of excitatory and inhibitory agents and a glial inhibitor on optically-recorded primary-afferent excitation.Mol Pain. 2008 Sep 26;4:39. doi: 10.1186/1744-8069-4-39. Mol Pain. 2008. PMID: 18817580 Free PMC article.
-
Presynaptic inhibition of transient receptor potential vanilloid type 1 (TRPV1) receptors by noradrenaline in nociceptive neurons.J Physiol. 2017 Apr 15;595(8):2639-2660. doi: 10.1113/JP273455. Epub 2017 Feb 22. J Physiol. 2017. PMID: 28094445 Free PMC article.
-
The role of spinal cord vanilloid (TRPV1) receptors in pain modulation.Physiol Res. 2008;57 Suppl 3:S69-S77. doi: 10.33549/physiolres.931601. Epub 2008 May 13. Physiol Res. 2008. PMID: 18481913 Review.
Cited by
-
Mas-related G-protein-coupled receptors inhibit pathological pain in mice.Proc Natl Acad Sci U S A. 2010 Sep 7;107(36):15933-8. doi: 10.1073/pnas.1011221107. Epub 2010 Aug 19. Proc Natl Acad Sci U S A. 2010. PMID: 20724664 Free PMC article.
-
Nonnociceptive afferent activity depresses nocifensive behavior and nociceptive synapses via an endocannabinoid-dependent mechanism.J Neurophysiol. 2013 Dec;110(11):2607-16. doi: 10.1152/jn.00170.2013. Epub 2013 Sep 11. J Neurophysiol. 2013. PMID: 24027102 Free PMC article.
-
MrgC agonism at central terminals of primary sensory neurons inhibits neuropathic pain.Pain. 2014 Mar;155(3):534-544. doi: 10.1016/j.pain.2013.12.008. Epub 2013 Dec 11. Pain. 2014. PMID: 24333779 Free PMC article.
-
Differential modulation of nociceptive versus non-nociceptive synapses by endocannabinoids.Mol Pain. 2013 Jun 1;9:26. doi: 10.1186/1744-8069-9-26. Mol Pain. 2013. PMID: 23725095 Free PMC article.
-
20:4-NAPE induced changes of mechanical sensitivity and DRG neurons excitability are concentration dependent and mediated via NAPE-PLD.Sci Rep. 2025 Apr 23;15(1):14131. doi: 10.1038/s41598-025-98567-y. Sci Rep. 2025. PMID: 40269193 Free PMC article.
References
-
- Yaksh TL, Farb DH, Leeman SE, Jessell TM. Intrathecal capsaicin depletes substance P in the rat spinal cord and produces prolonged thermal analgesia. Science. 1979;206:481–483. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

