Histone deacetylase inhibitor, trichostatin A induces ubiquitin-dependent cyclin D1 degradation in MCF-7 breast cancer cells
- PMID: 16504004
- PMCID: PMC1397858
- DOI: 10.1186/1476-4598-5-8
Histone deacetylase inhibitor, trichostatin A induces ubiquitin-dependent cyclin D1 degradation in MCF-7 breast cancer cells
Abstract
Background: Cyclin D1 is an important regulator of G1-S phase cell cycle transition and has been shown to be important for breast cancer development. GSK3beta phosphorylates cyclin D1 on Thr-286, resulting in enhanced ubiquitylation, nuclear export and degradation of the cyclin in the cytoplasm. Recent findings suggest that the development of small-molecule cyclin D1 ablative agents is of clinical relevance. We have previously shown that the histone deacetylase inhibitor trichostatin A (TSA) induces the rapid ubiquitin-dependent degradation of cyclin D1 in MCF-7 breast cancer cells prior to repression of cyclin D1 gene (CCND1) transcription. TSA treatment also resulted in accumulation of polyubiquitylated GFP-cyclin D1 species and reduced levels of the recombinant protein within the nucleus.
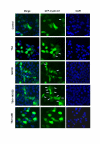
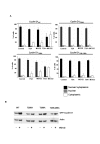
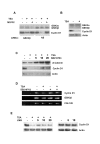
Results: Here we provide further evidence for TSA-induced ubiquitin-dependent degradation of cyclin D1 and demonstrate that GSK3beta-mediated nuclear export facilitates this activity. Our observations suggest that TSA treatment results in enhanced cyclin D1 degradation via the GSK3beta/CRM1-dependent nuclear export/26S proteasomal degradation pathway in MCF-7 cells.
Conclusion: We have demonstrated that rapid TSA-induced cyclin D1 degradation in MCF-7 cells requires GSK3beta-mediated Thr-286 phosphorylation and the ubiquitin-dependent 26S proteasome pathway. Drug induced cyclin D1 repression contributes to the inhibition of breast cancer cell proliferation and can sensitize cells to CDK and Akt inhibitors. In addition, anti-cyclin D1 therapy may be highly specific for treating human breast cancer. The development of potent and effective cyclin D1 ablative agents is therefore of clinical relevance. Our findings suggest that HDAC inhibitors may have therapeutic potential as small-molecule cyclin D1 ablative agents.
Figures

References
-
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. - PubMed
-
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. - PubMed
-
- Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

