Time to discontinuation of atypical versus typical antipsychotics in the naturalistic treatment of schizophrenia
- PMID: 16504026
- PMCID: PMC1402287
- DOI: 10.1186/1471-244X-6-8
Time to discontinuation of atypical versus typical antipsychotics in the naturalistic treatment of schizophrenia
Abstract
Background: There is an ongoing debate over whether atypical antipsychotics are more effective than typical antipsychotics in the treatment of schizophrenia. This naturalistic study compares atypical and typical antipsychotics on time to all-cause medication discontinuation, a recognized index of medication effectiveness in the treatment of schizophrenia.
Methods: We used data from a large, 3-year, observational, non-randomized, multisite study of schizophrenia, conducted in the U.S. between 7/1997 and 9/2003. Patients who were initiated on oral atypical antipsychotics (clozapine, olanzapine, risperidone, quetiapine, or ziprasidone) or oral typical antipsychotics (low, medium, or high potency) were compared on time to all-cause medication discontinuation for 1 year following initiation. Treatment group comparisons were based on treatment episodes using 3 statistical approaches (Kaplan-Meier survival analysis, Cox Proportional Hazards regression model, and propensity score-adjusted bootstrap resampling methods). To further assess the robustness of the findings, sensitivity analyses were performed, including the use of (a) only 1 medication episode for each patient, the one with which the patient was treated first, and (b) all medication episodes, including those simultaneously initiated on more than 1 antipsychotic.
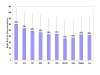
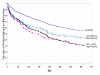
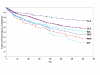
Results: Mean time to all-cause medication discontinuation was longer on atypical (N = 1132, 256.3 days) compared to typical antipsychotics (N = 534, 197.2 days; p < .01), and longer on atypicals compared to typicals of high potency (N = 320, 187.5 days; p < .01), medium potency (N = 140, 213.5 days; p < .01), and low potency (N = 74, 208.7 days; p < .01). Among the atypicals, only clozapine, olanzapine, and risperidone had significantly longer time to all-cause medication discontinuation compared to typicals, regardless of potency level, and compared to haloperidol with prophylactic anticholinergic treatment. When compared to perphenazine, a medium-potency typical antipsychotic, only clozapine and olanzapine had a consistently and significantly longer time to all-cause medication discontinuation. Results were confirmed by sensitivity analyses.
Conclusion: In the usual care of schizophrenia patients, time to medication discontinuation for any cause appears significantly longer for atypical than typical antipsychotics regardless of the typical antipsychotic potency level. Findings were primarily driven by clozapine and olanzapine, and to a lesser extent by risperidone. Furthermore, only clozapine and olanzapine therapy showed consistently and significantly longer treatment duration compared to perphenazine, a medium-potency typical antipsychotic.
Figures


References
-
- Herz MI, Glazer WM, Mostert MA, Sheard MA, Szymanski HV, Hafez H, Mirza M, Vana J. Intermittent vs. maintenance medication in schizophrenia: two-year results. Arch Gen Psychiatry. 1991;48:333–339. - PubMed
-
- Mojtabai R, Lavelle J, Gibson PJ, Bromet EJ. Atypical antipsychotics in first admission schizophrenia: medication continuation and outcomes. Schizophr Bull. 2003;29:519–530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

