Detection of the East and West African kdr mutation in Anopheles gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/Melt Curve analysis
- PMID: 16504072
- PMCID: PMC1409791
- DOI: 10.1186/1475-2875-5-16
Detection of the East and West African kdr mutation in Anopheles gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/Melt Curve analysis
Abstract
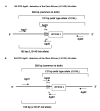
Background: Appropriate monitoring of vector resistance to insecticides is an integral component of planning and evaluation of insecticide use in malaria control programmes. The malaria vectors Anopheles gambiae s.s. and Anopheles arabiensis have developed resistance to pyrethroid insecticides as a result of a mechanism conferring reduced nervous system sensitivity, better known as knockdown resistance (kdr). In An. gambiae s.s. and An. arabiensis, two different substitutions in the para-type sodium channel, a L1014F substitution common in West Africa and a L1014S replacement found in Kenya, are linked with kdr. Two different allele-specific polymerase chain reactions (AS-PCR) are needed to detect these known kdr mutations. However, these AS-PCR assays rely on a single nucleotide polymorphism mismatch, which can result in unreliable results.
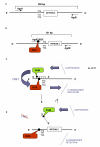
Methods: Here, a new assay for the detection of knockdown resistance in An. gambiae s.s. and An. arabiensis based on Fluorescence Resonance Energy Transfer/Melt Curve analysis (FRET/MCA) is presented and compared with the existing assays.
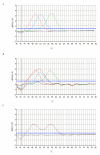
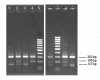
Results: The new FRET/MCA method has the important advantage of detecting both kdr alleles in one assay. Moreover, results show that the FRET/MCA is more reliable and more sensitive than the existing AS-PCR assays and is able to detect new genotypes. By using this technique, the presence of the East African kdr mutation (L1014S) is shown for the first time in An. arabiensis specimens from Uganda. In addition, a new kdr genotype is reported in An. gambiae s.s. from Uganda, where four An. gambiae s.s. mosquitoes possess both, the West (L1014F) and East (L1014S) African kdr allele, simultaneously.
Conclusion: The presence of both kdr mutations in the same geographical region shows the necessity of a reliable assay that enables to detect both mutations in one single assay. Hence, this new assay based on FRET/MCA will improve the screening of the kdr frequencies in An. gambiae s.s. and An. arabiensis.
Figures
References
-
- Lund AE, Narahashi T. Kinetics of sodium channel modification as the basis for the variation in the nerve membrane effects of pyrethroids and DDT analogs. Pestic Biochem Physiol . 1983;20:203–216. doi: 10.1016/0048-3575(83)90025-1. - DOI
-
- Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, Guillet P, Pasteur N, Pauron D. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–184. doi: 10.1046/j.1365-2583.1998.72062.x. - DOI - PubMed
-
- Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9:491–497. doi: 10.1046/j.1365-2583.2000.00209.x. - DOI - PubMed
-
- Diabaté A, Baldet T, Chandre F, Dabire KR, Simard F, Ouedraogo JB, Guillet P, Hougard JM. First report of a kdr mutation in Anopheles arabiensis from Burkina Faso, West Africa. J Am Mosq Control Assoc. 2004;20:195–196. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

