Airway smooth muscle relaxation results from a reduction in the frequency of Ca2+ oscillations induced by a cAMP-mediated inhibition of the IP3 receptor
- PMID: 16504084
- PMCID: PMC1459146
- DOI: 10.1186/1465-9921-7-34
Airway smooth muscle relaxation results from a reduction in the frequency of Ca2+ oscillations induced by a cAMP-mediated inhibition of the IP3 receptor
Abstract
Background: It has been shown that the contractile state of airway smooth muscle cells (SMCs) in response to agonists is determined by the frequency of Ca2+ oscillations occurring within the SMCs. Therefore, we hypothesized that the relaxation of airway SMCs induced by agents that increase cAMP results from the down-regulation or slowing of the frequency of the Ca2+ oscillations.
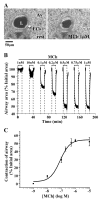
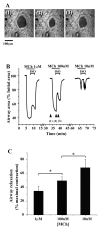
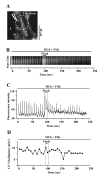
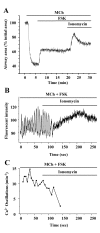
Methods: The effects of isoproterenol (ISO), forskolin (FSK) and 8-bromo-cAMP on the relaxation and Ca2+ signaling of airway SMCs contracted with methacholine (MCh) was investigated in murine lung slices with phase-contrast and laser scanning microscopy.
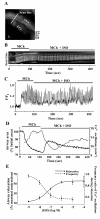
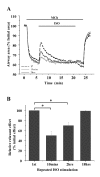
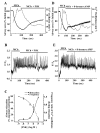
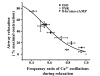
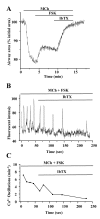
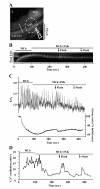
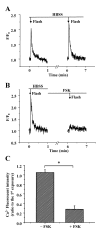
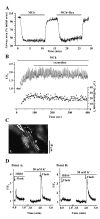
Results: All three cAMP-elevating agents simultaneously induced a reduction in the frequency of Ca2+ oscillations within the SMCs and the relaxation of contracted airways. The decrease in the Ca2+ oscillation frequency correlated with the extent of airway relaxation and was concentration-dependent. The mechanism by which cAMP reduced the frequency of the Ca2+ oscillations was investigated. Elevated cAMP did not affect the re-filling rate of the internal Ca2+ stores after emptying by repetitive exposure to 20 mM caffeine. Neither did elevated cAMP limit the Ca2+ available to stimulate contraction because an elevation of intracellular Ca2+ concentration induced by exposure to a Ca2+ ionophore (ionomycin) or by photolysis of caged-Ca2+ did not reverse the effect of cAMP. Similar results were obtained with iberiotoxin, a blocker of Ca2+-activated K+ channels, which would be expected to increase Ca2+ influx and contraction. By contrast, the photolysis of caged-IP3 in the presence of agonist, to further elevate the intracellular IP3 concentration, reversed the slowing of the frequency of the Ca2+ oscillations and relaxation of the airway induced by FSK. This result implied that the sensitivity of the IP3R to IP3 was reduced by FSK and this was supported by the reduced ability of IP3 to release Ca2+ in SMCs in the presence of FSK.
Conclusion: These results indicate that the relaxant effect of cAMP-elevating agents on airway SMCs is achieved by decreasing the Ca2+ oscillation frequency by reducing internal Ca2+ release through IP3 receptors.
Figures

References
-
- Global Initiative for Asthma. Global Strategy for asthma management and prevention. , National Institute of Health 2002, NIH publication no. 02-3659;
-
- McGrogan I, Lu S, Hipworth S, Sormaz L, Eng R, Preocanin D, Daniel EE. Mechanisms of cyclic nucleotide-induced relaxation in canine tracheal smooth muscle. Am J Physiol. 1995;268:L407–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

