Polarization of the endomembrane system is an early event in fucoid zygote development
- PMID: 16504093
- PMCID: PMC1397835
- DOI: 10.1186/1471-2229-6-5
Polarization of the endomembrane system is an early event in fucoid zygote development
Abstract
Background: Fucoid zygotes are excellent experimental organisms for investigating mechanisms that establish cell polarity and determine the site of tip growth. A common feature of polarity establishment is targeting endocytosis and exocytosis (secretion) to localized cortical domains. We have investigated the spatiotemporal development of endomembrane asymmetry in photopolarizing zygotes, and examined the underlying cellular physiology.
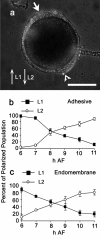
Results: The vital dye FM4-64 was used to visualize endomembranes. The endomembrane system preferentially accumulated at the rhizoid (growth) pole within 4 h of fertilization. The polarized endomembrane array was initially labile and reoriented when the developmental axis changed direction in response to changing light cues. Pharmacological studies indicated that vesicle trafficking, actin and microtubules were needed to maintain endomembrane polarity. In addition, endocytosis required a functional cortical actin cytoskeleton.
Conclusion: Endomembrane polarization is an early event in polarity establishment, beginning very soon after photolocalization of cortical actin to the presumptive rhizoid site. Targeting of endocytosis and secretion to the rhizoid cortex contributes to membrane asymmetry. We suggest that microtubule-actin interactions, possibly involving microtubule capture and stabilization at actin-rich sites in the rhizoid, may organize the endomembrane array.
Figures





Similar articles
-
Dynamic microtubules and endomembrane cycling contribute to polarity establishment and early development of Ectocarpus mitospores.Protoplasma. 2013 Oct;250(5):1035-43. doi: 10.1007/s00709-012-0476-5. Epub 2013 Jan 16. Protoplasma. 2013. PMID: 23322087
-
Establishment and expression of cellular polarity in fucoid zygotes.Microbiol Rev. 1992 Jun;56(2):316-39. doi: 10.1128/mr.56.2.316-339.1992. Microbiol Rev. 1992. PMID: 1620068 Free PMC article. Review.
-
Analyzing Rho GTPase-Dependent Processes During Cell Polarization in Brown Algae.Methods Mol Biol. 2018;1821:401-410. doi: 10.1007/978-1-4939-8612-5_27. Methods Mol Biol. 2018. PMID: 30062427
-
Asymmetric microtubule arrays organize the endoplasmic reticulum during polarity establishment in the brown alga Silvetia compressa.Cytoskeleton (Hoboken). 2010 Feb;67(2):102-11. doi: 10.1002/cm.20427. Cytoskeleton (Hoboken). 2010. PMID: 20169534
-
Choosing sides: establishment of polarity in zygotes of fucoid algae.Semin Cell Dev Biol. 2001 Oct;12(5):345-51. doi: 10.1006/scdb.2001.0262. Semin Cell Dev Biol. 2001. PMID: 11535041 Review.
Cited by
-
Dynamic microtubules and endomembrane cycling contribute to polarity establishment and early development of Ectocarpus mitospores.Protoplasma. 2013 Oct;250(5):1035-43. doi: 10.1007/s00709-012-0476-5. Epub 2013 Jan 16. Protoplasma. 2013. PMID: 23322087
-
Polarly localized WPR proteins interact with PAN receptors and the actin cytoskeleton during maize stomatal development.Plant Cell. 2023 Jan 2;35(1):469-487. doi: 10.1093/plcell/koac301. Plant Cell. 2023. PMID: 36227066 Free PMC article.
-
The Rac1 inhibitor, NSC23766, depolarizes adhesive secretion, endomembrane cycling, and tip growth in the fucoid alga, Silvetia compressa.Planta. 2008 Apr;227(5):991-1000. doi: 10.1007/s00425-007-0673-1. Epub 2008 Jan 9. Planta. 2008. PMID: 18183417
-
Rac1 signaling in the establishment of the fucoid algal body plan.Front Plant Sci. 2014 Dec 10;5:690. doi: 10.3389/fpls.2014.00690. eCollection 2014. Front Plant Sci. 2014. PMID: 25540648 Free PMC article.
-
Photopolarization of Fucus zygotes is determined by time sensitive vectorial addition of environmental cues during axis amplification.Front Plant Sci. 2015 Feb 3;6:26. doi: 10.3389/fpls.2015.00026. eCollection 2015. Front Plant Sci. 2015. PMID: 25691888 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

