Human sensory-evoked responses differ coincident with either "fusion-memory" or "flash-memory", as shown by stimulus repetition-rate effects
- PMID: 16504094
- PMCID: PMC1483834
- DOI: 10.1186/1471-2202-7-18
Human sensory-evoked responses differ coincident with either "fusion-memory" or "flash-memory", as shown by stimulus repetition-rate effects
Abstract
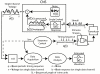
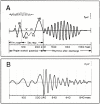
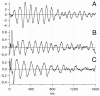
Background: A new method has been used to obtain human sensory evoked-responses whose time-domain waveforms have been undetectable by previous methods. These newly discovered evoked-responses have durations that exceed the time between the stimuli in a continuous stream, thus causing an overlap which, up to now, has prevented their detection. We have named them "A-waves", and added a prefix to show the sensory system from which the responses were obtained (visA-waves, audA-waves, somA-waves).
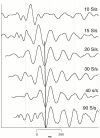
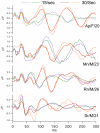
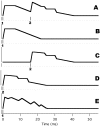
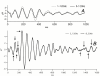
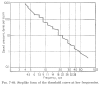
Results: When A-waves were studied as a function of stimulus repetition-rate, it was found that there were systematic differences in waveshape at repetition-rates above and below the psychophysical region in which the sensation of individual stimuli fuse into a continuity. The fusion phenomena is sometimes measured by a "Critical Fusion Frequency", but for this research we can only identify a frequency-region [which we call the STZ (Sensation-Transition Zone)]. Thus, the A-waves above the STZ differed from those below the STZ, as did the sensations. Study of the psychophysical differences in auditory and visual stimuli, as shown in this paper, suggest that different stimulus features are detected, and remembered, at stimulation rates above and below STZ.
Conclusion: The results motivate us to speculate that: 1) Stimulus repetition-rates above the STZ generate waveforms which underlie "fusion-memory" whereas rates below the STZ show neuronal processing in which "flash-memory" occurs. 2) These two memories differ in both duration and mechanism, though they may occur in the same cell groups. 3) The differences in neuronal processing may be related to "figure" and "ground" differentiation. We conclude that A-waves provide a novel measure of neural processes that can be detected on the human scalp, and speculate that they may extend clinical applications of evoked response recordings. If A-waves also occur in animals, it is likely that A-waves will provide new methods for comparison of activity of neuronal populations and single cells.
Figures


















Similar articles
-
Effects of visual and auditory stimuli on median nerve somatosensory evoked potentials in man.Electromyogr Clin Neurophysiol. 1995 Jun-Jul;35(4):251-6. Electromyogr Clin Neurophysiol. 1995. PMID: 7555931
-
The use of sensory evoked potentials in toxicology.Fundam Appl Toxicol. 1985 Feb;5(1):24-40. Fundam Appl Toxicol. 1985. PMID: 3886466 Review.
-
Sensory evoked potentials in Creutzfeldt-Jakob disease.Eur Neurol. 1990;30(3):157-61. doi: 10.1159/000117335. Eur Neurol. 1990. PMID: 2192888
-
Auditory brainstem implant: electrophysiologic responses and subject perception.Ear Hear. 2015 May-Jun;36(3):368-76. doi: 10.1097/AUD.0000000000000126. Ear Hear. 2015. PMID: 25437141 Free PMC article.
-
A review of plasticity induced by auditory and visual tetanic stimulation in humans.Eur J Neurosci. 2018 Aug;48(4):2084-2097. doi: 10.1111/ejn.14080. Epub 2018 Aug 10. Eur J Neurosci. 2018. PMID: 30025183 Review.
Cited by
-
Continuous- and discrete-time stimulus sequences for high stimulus rate paradigm in evoked potential studies.Comput Math Methods Med. 2013;2013:396034. doi: 10.1155/2013/396034. Epub 2013 Mar 31. Comput Math Methods Med. 2013. PMID: 23606900 Free PMC article.
References
-
- Kompass R. Universal temporal structures in human information processing: a neural principle and psychophysical evidence. In: Kaernback C, Schroger E, Muller H, editor. Psychophysics Beyond Sensation: Laws and Invariants of Human Cognition. Mahwah, NJ: Lawrence Erlbaum Associates; 2004. pp. 451–480.
-
- Lalanne L. Sur la duree de la sensation tactile [On the duration of tactile sensations] Note Comptes Rendus de l'Acadenue des Sciences Paris. 1876;39:1314–1316.
-
- Brecher GA. The emergence and biological significance of the subjective time unit perceptual moment. (in German: Die Entstehung und biologische Bedeutung der subjektiven Zeiteinheit – des Moments. Zeitschrift fur vergleichende Physiologie. 1932;18:204–243.
-
- Maskin MB, Riklan M, Chabot D. Effects of "short-term" versus "long-term" L-Dopa therapy in parkinsonism on critical flicker frequency. Percept Mot Skills. 1974;38:455–458. - PubMed
-
- Riklan M, Levita E, Misiak H. Critical flicker frequency and integrative functions in parkinsonism. J Psychol. 1970;75:45–51. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

