High resolution ultrasound-guided microinjection for interventional studies of early embryonic and placental development in vivo in mice
- PMID: 16504164
- PMCID: PMC1436015
- DOI: 10.1186/1471-213X-6-10
High resolution ultrasound-guided microinjection for interventional studies of early embryonic and placental development in vivo in mice
Abstract
Background: In utero microinjection has proven valuable for exploring the developmental consequences of altering gene expression, and for studying cell lineage or migration during the latter half of embryonic mouse development (from embryonic day 9.5 of gestation (E9.5)). In the current study, we use ultrasound guidance to accurately target microinjections in the conceptus at E6.5-E7.5, which is prior to cardiovascular or placental dependence. This method may be useful for determining the developmental effects of targeted genetic or cellular interventions at critical stages of placentation, gastrulation, axis formation, and neural tube closure.
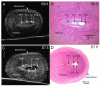
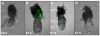
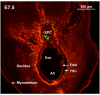
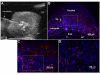
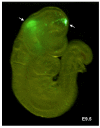
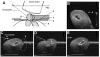
Results: In 40 MHz ultrasound images at E6.5, the ectoplacental cone region and proamniotic cavity could be visualized. The ectoplacental cone region was successfully targeted with 13.8 nL of a fluorescent bead suspension with few or no beads off-target in 51% of concepti microinjected at E6.5 (28/55 injected). Seventy eight percent of the embryos survived 2 to 12 days post injection (93/119), 73% (41/56) survived to term of which 68% (38/56) survived and appeared normal one week after birth. At E7.5, the amniotic and exocoelomic cavities, and ectoplacental cone region were discernable. Our success at targeting with few or no beads off-target was 90% (36/40) for the ectoplacental cone region and 81% (35/43) for the exocoelomic cavity but tended to be less, 68% (34/50), for the smaller amniotic cavity. At E11.5, beads microinjected at E7.5 into the ectoplacental cone region were found in the placental spongiotrophoblast layer, those injected into the exocoelomic cavity were found on the surface or within the placental labyrinth, and those injected into the amniotic cavity were found on the surface or within the embryo. Following microinjection at E7.5, survival one week after birth was 60% (26/43) when the amniotic cavity was the target and 66% (19/29) when the target was the ectoplacental cone region. The survival rate was similar in sham experiments, 54% (33/61), for which procedures were identical but no microinjection was performed, suggesting that surgery and manipulation of the uterus were the main causes of embryonic death.
Conclusion: Ultrasound-guided microinjection into the ectoplacental cone region at E6.5 or E7.5 and the amniotic cavity at E7.5 was achieved with a 7 day postnatal survival of >/=60%. Target accuracy of these sites and of the exocoelomic cavity at E7.5 was > or =51%. We suggest that this approach may be useful for exploring gene function during early placental and embryonic development.
Figures



References
-
- Weiner HL, Bakst R, Hurlbert MS, Ruggiero J, Ahn E, Lee WS, Stephen D, Zagzag D, Joyner AL, Turnbull DH. Induction of medulloblastomas in mice by sonic hedgehog, independent of Gli1. Cancer Res. 2002;62:6385–6389. - PubMed
-
- Holzinger A, Trapnell BC, Weaver TE, Whitsett JA, Iwamoto HS. Intraamniotic administration of an adenoviral vector for gene transfer to fetal sheep and mouse tissues. Pediatr Res. 1995;38:844–850. - PubMed
-
- Christensen G, Minamisawa S, Gruber PJ, Wang Y, Chien KR. High-efficiency, long-term cardiac expression of foreign genes in living mouse embryos and neonates. Circulation. 2000;101:178–184. - PubMed
-
- Senoo M, Matsubara Y, Fujii K, Nagasaki Y, Hiratsuka M, Kure S, Uehara S, Okamura K, Yajima A, Narisawa K. Adenovirus-mediated in utero gene transfer in mice and guinea pigs: tissue distribution of recombinant adenovirus determined by quantitative TaqMan-polymerase chain reaction assay. Mol Genet Metab. 2000;69:269–276. doi: 10.1006/mgme.2000.2984. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

