The primary defect in experimental ileitis originates from a nonhematopoietic source
- PMID: 16505137
- PMCID: PMC2118253
- DOI: 10.1084/jem.20050407
The primary defect in experimental ileitis originates from a nonhematopoietic source
Abstract
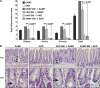
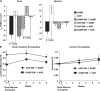
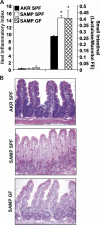
The initiating etiologic factor in Crohn's disease (CD) remains unclear. SAMP1/YitFc (SAMP) mice develop chronic ileitis similar to human CD. We used bone marrow chimeras to determine if SAMP ileitis results from a primary immunological defect or from dysregulated mucosal immunity secondary to intrinsic, nonhematopoietic (e.g., epithelial) dysfunction. SAMP mice receiving wild-type (AKR) BM developed severe ileitis, whereas SAMP BM did not confer ileitis to WT recipients. WT lymphocytes from reconstituted SAMP mice resembled native SAMP populations in regard to surface phenotype and cytokine production. Ilea from native SAMP mice and SAMP recipients of wild-type BM displayed decreased epithelial barrier resistance ex vivo and increased epithelial permeability in vivo compared to native WT mice and AKR recipients of SAMP BM. This permeability defect preceded the development of ileal inflammation, was present in the absence of commensal bacteria, and was accompanied by altered ileal mRNA expression of the tight junction proteins claudin-2 and occludin. Our results provide evidence that the primary defect conferring ileitis in SAMP mice originates from a nonhematopoietic source. Generation of pathogenic lymphocytes is a consequence of this defect and does not reflect intrinsic proinflammatory leukocyte properties. Decreased barrier function suggests that defects in the epithelium may represent the primary source of SAMP ileitis susceptibility.
Figures




References
-
- Bouma, G., and W. Strober. 2003. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 3:521–533. - PubMed
-
- Egan, L.J., and W.J. Sandborn. 2004. Advances in the treatment of Crohn's disease. Gastroenterology. 126:1574–1581. - PubMed
-
- Ma, T.Y. 1997. Intestinal epithelial barrier dysfunction in Crohn's disease. Proc. Soc. Exp. Biol. Med. 214:318–327. - PubMed
-
- Shanahan, F. 2002. Crohn's disease. Lancet. 359:62–69. - PubMed
-
- Ogura, Y., N. Inohara, A. Benito, F.F. Chen, S. Yamaoka, and G. Nunez. 2001. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J. Biol. Chem. 276:4812–4818. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

