Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula toxin psalmotoxin 1 is state dependent
- PMID: 16505147
- PMCID: PMC2151504
- DOI: 10.1085/jgp.200509409
Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula toxin psalmotoxin 1 is state dependent
Abstract
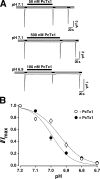
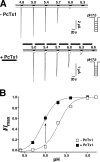
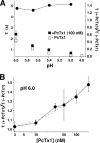
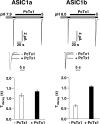
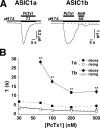
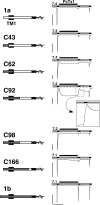
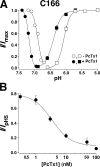
Acid-sensing ion channels (ASICs) are Na(+) channels gated by extracellular H(+). Six ASIC subunits that are expressed in neurons have been characterized. The tarantula toxin psalmotoxin 1 has been reported to potently and specifically inhibit homomeric ASIC1a and has been useful to characterize ASICs in neurons. Recently we have shown that psalmotoxin 1 inhibits ASIC1a by increasing its apparent affinity for H(+). However, the mechanism by which PcTx1 increases the apparent H(+) affinity remained unclear. Here we show that PcTx1 also interacts with ASIC1b, a splice variant of ASIC1a. However, PcTx1 does not inhibit ASIC1b but promotes its opening; under slightly acidic conditions, PcTx1 behaves like an agonist for ASIC1b. Our results are most easily explained by binding of PcTx1 with different affinities to different states (closed, open, and desensitized) of the channel. For ASIC1b, PcTx1 binds most tightly to the open state, promoting opening, whereas for ASIC1a, it binds most tightly to the open and the desensitized state, promoting desensitization.
Figures

References
-
- Babini, E., M. Paukert, H.S. Geisler, and S. Gründer. 2002. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1). J. Biol. Chem. 277:41597–41603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

