A motor neuron disease-associated mutation in p150Glued perturbs dynactin function and induces protein aggregation
- PMID: 16505168
- PMCID: PMC2063705
- DOI: 10.1083/jcb.200511068
A motor neuron disease-associated mutation in p150Glued perturbs dynactin function and induces protein aggregation
Abstract
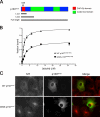
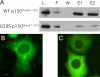
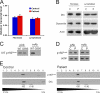
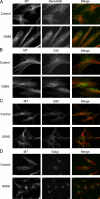
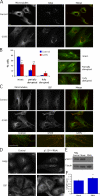
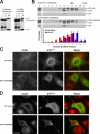
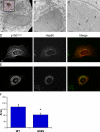
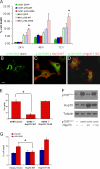
The microtubule motor cytoplasmic dynein and its activator dynactin drive vesicular transport and mitotic spindle organization. Dynactin is ubiquitously expressed in eukaryotes, but a G59S mutation in the p150Glued subunit of dynactin results in the specific degeneration of motor neurons. This mutation in the conserved cytoskeleton-associated protein, glycine-rich (CAP-Gly) domain lowers the affinity of p150Glued for microtubules and EB1. Cell lines from patients are morphologically normal but show delayed recovery after nocodazole treatment, consistent with a subtle disruption of dynein/dynactin function. The G59S mutation disrupts the folding of the CAP-Gly domain, resulting in aggregation of the p150Glued protein both in vitro and in vivo, which is accompanied by an increase in cell death in a motor neuron cell line. Overexpression of the chaperone Hsp70 inhibits aggregate formation and prevents cell death. These data support a model in which a point mutation in p150Glued causes both loss of dynein/dynactin function and gain of toxic function, which together lead to motor neuron cell death.
Figures
References
-
- Barral, J.M., S.A. Broadley, G. Schaffar, and F.U. Hartl. 2004. Roles of molecular chaperones in protein misfolding diseases. Semin. Cell Dev. Biol. 15:17–29. - PubMed
-
- Brooks, B.P., D.E. Merry, H.L. Paulson, A.P. Lieberman, D.L. Kolson, and K.H. Fischbeck. 1998. A cell culture model for androgen effects in motor neurons. J. Neurochem. 70:1054–1060. - PubMed
-
- Bruijn, L.I., T.M. Miller, and D.W. Cleveland. 2004. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 27:723–749. - PubMed
-
- Carson, J.H., H. Cui, and E. Barbarese. 2001. The balance of power in RNA trafficking. Curr. Opin. Neurobiol. 11:558–563. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

