A DEAD-box protein alone promotes group II intron splicing and reverse splicing by acting as an RNA chaperone
- PMID: 16505350
- PMCID: PMC1450124
- DOI: 10.1073/pnas.0600332103
A DEAD-box protein alone promotes group II intron splicing and reverse splicing by acting as an RNA chaperone
Abstract
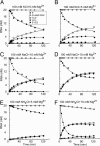
Group II intron RNAs self-splice in vitro but only at high salt and/or Mg2+ concentrations and have been thought to require proteins to stabilize their active structure for efficient splicing in vivo. Here, we show that a DEAD-box protein, CYT-19, can by itself promote the splicing and reverse splicing of the yeast aI5gamma and bI1 group II introns under near-physiological conditions by acting as an ATP-dependent RNA chaperone, whose continued presence is not required after RNA folding. Our results suggest that the folding of some group II introns may be limited by kinetic traps and that their active structures, once formed, do not require proteins or high Mg2+ concentrations for structural stabilization. Thus, during evolution, group II introns could have spliced and transposed by reverse splicing by using ubiquitous RNA chaperones before acquiring more specific protein partners to promote their splicing and mobility. More generally, our results provide additional evidence for the widespread role of RNA chaperones in folding cellular RNAs.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures




References
-
- Michel M., Ferat J. L. Biochemistry. 1995;64:435–461. - PubMed
-
- Lehmann K., Schmidt U. Crit. Rev. Biochem. Mol. Biol. 2003;38:249–303. - PubMed
-
- Pyle A. M., Lambowitz A. M. In: The RNA World. 3rd Ed. Gesteland R. F., Atkins J. F., Cech T. R., editors. Plainview, NY: Cold Spring Harbor Lab Press; 2006. pp. 469–505.
-
- Lambowitz A. M., Caprara M. G., Zimmerly S., Perlman P. S. In: The RNA World. 2nd Ed. Gesteland R. F., Atkins J. F., Cech T. R., editors. Plainview, NY: Cold Spring Harbor Lab Press; 1999. pp. 451–485.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

