An elongated spine of buried core residues necessary for in vivo folding of the parallel beta-helix of P22 tailspike adhesin
- PMID: 16505375
- PMCID: PMC1383501
- DOI: 10.1073/pnas.0509087103
An elongated spine of buried core residues necessary for in vivo folding of the parallel beta-helix of P22 tailspike adhesin
Abstract
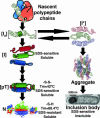
The parallel beta-helix is an elongated beta-sheet protein domain associated with microbial virulence factors, toxins, viral adhesins, and allergens. Long stacks of similar, buried residues are a prominent feature of this fold, as well as the polypeptide chain fold of an amyloid structure. The 13-rung, right-handed, parallel beta-helix of the homotrimeric P22 tailspike adhesin exhibits predominantly hydrophobic stacks. The role of these stacked residues in the folding and stabilization of the protein is unclear. Through scanning alanine mutagenesis we have identified a folding spine of stacked residues in continuous contact along the length of P22 tailspike's beta-helix domain that is necessary for folding within cells. Nearly all chains carrying alanine substitutions of the 103 buried nonalanines were defective in folding in vivo at 37 degrees C. However, the majority of these chains successfully reached a native state, stable to >80 degrees C, when folded inside cells at low temperatures. Thus, nearly the entire buried core was critical for in vivo beta-helix folding but negligible for stability. Folding at 18 degrees C revealed the minimal folding spine of 29 nonglycine stack positions that were intolerant to alanine substitution. These results indicate that a processive folding mechanism, dependent on stacking contacts, controls beta-helix formation. Such a stepwise folding pathway offers a new target for drug design against this class of microbial virulence factors.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures




Similar articles
-
Buried hydrophobic side-chains essential for the folding of the parallel beta-helix domains of the P22 tailspike.Protein Sci. 2004 Sep;13(9):2291-303. doi: 10.1110/ps.04676704. Protein Sci. 2004. PMID: 15322277 Free PMC article.
-
Stalled folding mutants in the triple beta-helix domain of the phage P22 tailspike adhesin.J Mol Biol. 2005 Dec 16;354(5):1103-17. doi: 10.1016/j.jmb.2005.10.007. Epub 2005 Oct 27. J Mol Biol. 2005. PMID: 16289113
-
Plasticity and steric strain in a parallel beta-helix: rational mutations in the P22 tailspike protein.Proteins. 2000 Apr 1;39(1):89-101. Proteins. 2000. PMID: 10737931
-
There's a right way and a wrong way: in vivo and in vitro folding, misfolding and subunit assembly of the P22 tailspike.Structure. 1999 Jun 15;7(6):R131-9. doi: 10.1016/s0969-2126(99)80078-1. Structure. 1999. PMID: 10404587 Review.
-
Phage tailspike protein. A fishy tale of protein folding.Curr Biol. 1994 Nov 1;4(11):1026-9. doi: 10.1016/s0960-9822(00)00234-7. Curr Biol. 1994. PMID: 7874487 Review.
Cited by
-
The C-terminal cysteine annulus participates in auto-chaperone function for Salmonella phage P22 tailspike folding and assembly.Bacteriophage. 2012 Jan 1;2(1):36-49. doi: 10.4161/bact.19775. Bacteriophage. 2012. PMID: 22666655 Free PMC article.
-
Stepwise folding of an autotransporter passenger domain is not essential for its secretion.J Biol Chem. 2013 Dec 6;288(49):35028-38. doi: 10.1074/jbc.M113.515635. Epub 2013 Oct 28. J Biol Chem. 2013. PMID: 24165126 Free PMC article.
-
PfbA, a novel plasmin- and fibronectin-binding protein of Streptococcus pneumoniae, contributes to fibronectin-dependent adhesion and antiphagocytosis.J Biol Chem. 2008 Dec 26;283(52):36272-9. doi: 10.1074/jbc.M807087200. Epub 2008 Oct 30. J Biol Chem. 2008. PMID: 18974092 Free PMC article.
-
Hydrophobic core mutations associated with cataract development in mice destabilize human gammaD-crystallin.J Biol Chem. 2009 Nov 27;284(48):33285-95. doi: 10.1074/jbc.M109.031344. Epub 2009 Sep 16. J Biol Chem. 2009. PMID: 19758984 Free PMC article.
-
A role for amyloid in cell aggregation and biofilm formation.PLoS One. 2011 Mar 8;6(3):e17632. doi: 10.1371/journal.pone.0017632. PLoS One. 2011. PMID: 21408122 Free PMC article.
References
-
- Jenkins J., Pickersgill R. Prog. Biophys. Mol. Biol. 2001;77:111–175. - PubMed
-
- Emsley P., Charles I. G., Fairweather N. F., Isaacs N. W. Nature. 1996;381:90–92. - PubMed
-
- Hegde S. S., Vetting M. W., Roderick S. L., Mitchenall L. A., Maxwell A., Takiff H. E., Blanchard J. S. Science. 2005;308:1480–1483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

