Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment
- PMID: 16505384
- PMCID: PMC1450149
- DOI: 10.1073/pnas.0506977103
Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment
Abstract
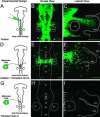
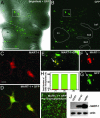
Human metastatic melanoma cells express a dedifferentiated, plastic phenotype, which may serve as a selective advantage, because melanoma cells invade various microenvironments. Over the last three decades, there has been an increased focus on the role of the tumor microenvironment in cancer progression, with the goal of reversing the metastatic phenotype. Here, using an embryonic chick model, we explore the possibility of reverting the metastatic melanoma phenotype to its cell type of origin, the neural-crest-derived melanocyte. GFP-labeled adult human metastatic melanoma cells were transplanted in ovo adjacent to host chick premigratory neural crest cells and analyzed 48 and 96 h after egg reincubation. Interestingly, the transplanted melanoma cells do not form tumors. Instead, we find that transplanted melanoma cells invade surrounding chick tissues in a programmed manner, distributing along host neural-crest-cell migratory pathways. The invading melanoma cells display neural-crest-cell-like morphologies and populate host peripheral structures, including the branchial arches, dorsal root and sympathetic ganglia. Analysis of a melanocyte-specific phenotype marker (MART-1) and a neuronal marker (Tuj1) revealed a subpopulation of melanoma cells that invade the chick periphery and express MART-1 and Tuj1. Our results demonstrate the ability of adult human metastatic melanoma cells to respond to chick embryonic environmental cues, a subset of which may undergo a reprogramming of their metastatic phenotype. This model has the potential to provide insights into the regulation of tumor cell plasticity by an embryonic milieu, which may hold significant therapeutic promise.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



Similar articles
-
Neural crest cell migration of mouse B16-F1 melanoma cells transplanted into the chick embryo is inhibited by the BMP-antagonist noggin.Int J Oncol. 2007 Dec;31(6):1367-78. Int J Oncol. 2007. PMID: 17982664
-
[Phenotypic plasticity of neural crest-derived melanocytes and Schwann cells].Biol Aujourdhui. 2011;205(1):53-61. doi: 10.1051/jbio/2011008. Epub 2011 Apr 19. Biol Aujourdhui. 2011. PMID: 21501576 Review. French.
-
Migratory patterns of sympathetic ganglioblasts and other neural crest derivatives in chick embryos.J Neurosci. 1986 Dec;6(12):3465-73. doi: 10.1523/JNEUROSCI.06-12-03465.1986. J Neurosci. 1986. PMID: 3794784 Free PMC article.
-
Imaging neural crest cell dynamics during formation of dorsal root ganglia and sympathetic ganglia.Development. 2005 Jan;132(2):235-45. doi: 10.1242/dev.01553. Epub 2004 Dec 8. Development. 2005. PMID: 15590743
-
Reprogramming metastatic tumour cells with embryonic microenvironments.Nat Rev Cancer. 2007 Apr;7(4):246-55. doi: 10.1038/nrc2108. Nat Rev Cancer. 2007. PMID: 17384580 Review.
Cited by
-
Tumorigenic potential is restored during differentiation in fusion-reprogrammed cancer cells.Cell Death Dis. 2016 Jul 28;7(7):e2314. doi: 10.1038/cddis.2016.189. Cell Death Dis. 2016. PMID: 27468690 Free PMC article.
-
The hormetic morphogen theory of curvature and the morphogenesis and pathology of tubular and other curved structures.Dose Response. 2009 Oct 16;7(4):307-31. doi: 10.2203/dose-response.09-013.Fosslien. Dose Response. 2009. PMID: 20011651 Free PMC article.
-
Stem-Cell Theory of Cancer: Implications for Antiaging and Anticancer Strategies.Cancers (Basel). 2022 Mar 4;14(5):1338. doi: 10.3390/cancers14051338. Cancers (Basel). 2022. PMID: 35267646 Free PMC article.
-
Zebrafish embryo extracts promote sphere-forming abilities of human melanoma cell line.Cancer Sci. 2009 Aug;100(8):1429-33. doi: 10.1111/j.1349-7006.2009.01218.x. Epub 2009 May 18. Cancer Sci. 2009. PMID: 19522854 Free PMC article.
-
Reprogramming Malignant Cancer Cells toward a Benign Phenotype following Exposure to Human Embryonic Stem Cell Microenvironment.PLoS One. 2017 Jan 9;12(1):e0169899. doi: 10.1371/journal.pone.0169899. eCollection 2017. PLoS One. 2017. PMID: 28068409 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

