Farnesylation or geranylgeranylation? Efficient assays for testing protein prenylation in vitro and in vivo
- PMID: 16507103
- PMCID: PMC1448197
- DOI: 10.1186/1471-2091-7-6
Farnesylation or geranylgeranylation? Efficient assays for testing protein prenylation in vitro and in vivo
Abstract
Background: Available in vitro and in vivo methods for verifying protein substrates for posttranslational modifications via farnesylation or geranylgeranylation (for example, autoradiography with 3H-labeled anchor precursors) are time consuming (weeks/months), laborious and suffer from low sensitivity.
Results: We describe a new technique for detecting prenyl anchors in N-terminally glutathione S-transferase (GST)-labeled constructs of target proteins expressed in vitro in rabbit reticulocyte lysate and incubated with 3H-labeled anchor precursors. Alternatively, hemagglutinin (HA)-labeled constructs expressed in vivo (in cell culture) can be used. For registration of the radioactive marker, we propose to use a thin layer chromatography (TLC) analyzer. As a control, the protein yield is tested by Western blotting with anti-GST- (or anti-HA-) antibodies on the same membrane that has been previously used for TLC-scanning. These protocols have been tested with Rap2A, v-Ki-Ras2 and RhoA (variant RhoA63L) including the necessary controls. We show directly that RasD2 is a farnesylation target.
Conclusion: Savings in time for experimentation and the higher sensitivity for detecting 3H-labeled lipid anchors recommend the TLC-scanning method with purified GST- (or HA-) tagged target proteins as the method of choice for analyzing their prenylation capabilities in vitro and in vivo and, possibly, also for studying the myristoyl and palmitoyl posttranslational modifications.
Figures
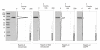

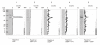
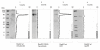

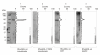



Similar articles
-
Measurement of protein farnesylation and geranylgeranylation in vitro, in cultured cells and in biopsies, and the effects of prenyl transferase inhibitors.Nat Protoc. 2011 Oct 27;6(11):1775-91. doi: 10.1038/nprot.2011.387. Nat Protoc. 2011. PMID: 22036881 Free PMC article.
-
Protein prenylation and human diseases: a balance of protein farnesylation and geranylgeranylation.Sci China Life Sci. 2015 Apr;58(4):328-35. doi: 10.1007/s11427-015-4836-1. Epub 2015 Apr 11. Sci China Life Sci. 2015. PMID: 25862656 Review.
-
Prenylated proteins in regenerating rat liver.Biochem Biophys Res Commun. 1994 May 16;200(3):1713-20. doi: 10.1006/bbrc.1994.1650. Biochem Biophys Res Commun. 1994. PMID: 8185630
-
In vitro prenylation assay of Arabidopsis proteins.Methods Mol Biol. 2013;1043:147-60. doi: 10.1007/978-1-62703-532-3_16. Methods Mol Biol. 2013. PMID: 23913045
-
Protein farnesylation: implications for normal physiology, malignant transformation, and cancer therapy.Cancer Cell. 2005 Apr;7(4):297-300. doi: 10.1016/j.ccr.2005.04.005. Cancer Cell. 2005. PMID: 15837619 Review.
Cited by
-
A novel testis-specific GTPase serves as a link to proteasome biogenesis: functional characterization of RhoS/RSA-14-44 in spermatogenesis.Mol Biol Cell. 2010 Dec;21(24):4312-24. doi: 10.1091/mbc.E10-04-0310. Epub 2010 Oct 27. Mol Biol Cell. 2010. PMID: 20980621 Free PMC article.
-
Protective effects of intrathecal injection of AAV9-RabGGTB-GFP+ in SOD1G93A mice.Front Aging Neurosci. 2023 Mar 14;15:1092607. doi: 10.3389/fnagi.2023.1092607. eCollection 2023. Front Aging Neurosci. 2023. PMID: 36967828 Free PMC article.
-
Transcriptome analysis of finger millet (Eleusine coracana (L.) Gaertn.) reveals unique drought responsive genes.J Genet. 2019 Jun;98(2):46. J Genet. 2019. PMID: 31204698
-
Direct spatial control of Epac1 by cyclic AMP.Mol Cell Biol. 2009 May;29(10):2521-31. doi: 10.1128/MCB.01630-08. Epub 2009 Mar 9. Mol Cell Biol. 2009. PMID: 19273589 Free PMC article.
-
A novel approach to tag and identify geranylgeranylated proteins.Electrophoresis. 2009 Oct;30(20):3598-606. doi: 10.1002/elps.200900259. Electrophoresis. 2009. PMID: 19784953 Free PMC article.
References
-
- Moores SL, Schaber MD, Mosser SD, Rands E, O'Hara MB, Garsky VM, Marshall MS, Pompliano DL, Gibbs JB. Sequence dependence of protein isoprenylation. J Biol Chem. 1991;266:14603–14610. - PubMed
-
- Caplin BE, Hettich LA, Marshall MS. Substrate characterization of the Saccharomyces cerevisiae protein farnesyltransferase and type-I protein geranylgeranyltransferase. Biochim Biophys Acta. 1994;1205:39–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

