Continuous infusion of ceftazidime in critically ill patients undergoing continuous venovenous haemodiafiltration: pharmacokinetic evaluation and dose recommendation
- PMID: 16507147
- PMCID: PMC1550796
- DOI: 10.1186/cc3993
Continuous infusion of ceftazidime in critically ill patients undergoing continuous venovenous haemodiafiltration: pharmacokinetic evaluation and dose recommendation
Abstract
Introduction: In seriously infected patients with acute renal failure and who require continuous renal replacement therapy, data on continuous infusion of ceftazidime are lacking. Here we analyzed the pharmacokinetics of ceftazidime administered by continuous infusion in critically ill patients during continuous venovenous haemodiafiltration (CVVHDF) in order to identify the optimal dosage in this setting.
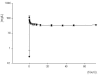
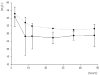
Method: Seven critically ill patients were prospectively enrolled in the study. CVVHDF was performed using a 0.6 m2 AN69 high-flux membrane and with blood, dialysate and ultrafiltration flow rates of 150 ml/min, 1 l/hour and 1.5 l/hour, respectively. Based on a predicted haemodiafiltration clearance of 32.5 ml/min, all patients received a 2 g loading dose of ceftazidime, followed by a 3 g/day continuous infusion for 72 hours. Serum samples were collected at 0, 3, 15 and 30 minutes and at 1, 2, 4, 6, 8, 12, 24, 36, 48 and 72 hours; dialysate/ultrafiltrate samples were taken at 2, 8, 12, 24, 36 and 48 hours. Ceftazidime concentrations in serum and dialysate/ultrafiltrate were measured using high-performance liquid chromatography.
Results: The mean (+/- standard deviation) elimination half-life, volume of distribution, area under the concentration-time curve from time 0 to 72 hours, and total clearance of ceftazidime were 4 +/- 1 hours, 19 +/- 6 l, 2514 +/- 212 mg/h per l, and 62 +/- 5 ml/min, respectively. The mean serum ceftazidime steady-state concentration was 33.5 mg/l (range 28.8-36.3 mg/l). CVVHDF effectively removed continuously infused ceftazidime, with a sieving coefficient and haemodiafiltration clearance of 0.81 +/- 0.11 and 33.6 +/- 4 mg/l, respectively.
Conclusion: We conclude that a dosing regimen of 3 g/day ceftazidime, by continuous infusion, following a 2 g loading dose, results in serum concentrations more than four times the minimum inhibitory concentration for all susceptible pathogens, and we recommend this regimen in critically ill patients undergoing CVVHDF.
Figures
References
-
- Mouton JW, Vinks AA. Is continuous infusion of β-lactam antibiotics worthwile? Efficacy and pharmacokinetic considerations. J Antimicrob Chemother. 1996;38:5–15. - PubMed
-
- Angus BJ, Smith MD, Suputtamongkol Y, Mattie H, Walsh AL, Wuthiekanun V, Chaowagul W, White NJ. Pharmacokinetic-pharmacodynamic evaluation of cefatzidime continuous infusion vs intermittent bolus injection in septicaemic melioidosis. Br J Clin Pharmacol. 2000;50:184–191. doi: 10.1046/j.1365-2125.2000.00179.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

