Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites
- PMID: 16507167
- PMCID: PMC1431722
- DOI: 10.1186/gb-2006-7-2-r12
Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites
Abstract
Background: The apicomplexan parasite Plasmodium falciparum causes the most severe form of malaria in humans. After invasion into erythrocytes, asexual parasite stages drastically alter their host cell and export remodeling and virulence proteins. Previously, we have reported identification and functional analysis of a short motif necessary for export of proteins out of the parasite and into the red blood cell.
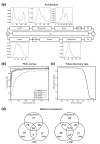
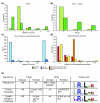
Results: We have developed software for the prediction of exported proteins in the genus Plasmodium, and identified exported proteins conserved between malaria parasites infecting rodents and the two major causes of human malaria, P. falciparum and P. vivax. This conserved 'exportome' is confined to a few subtelomeric chromosomal regions in P. falciparum and the synteny of these and surrounding regions is conserved in P. vivax. We have identified a novel gene family PHIST (for Plasmodium helical interspersed subtelomeric family) that shares a unique domain with 72 paralogs in P. falciparum and 39 in P. vivax; however, there is only one member in each of the three species studied from the P. berghei lineage.
Conclusion: These data suggest radiation of genes encoding remodeling and virulence factors from a small number of loci in a common Plasmodium ancestor, and imply a closer phylogenetic relationship between the P. vivax and P. falciparum lineages than previously believed. The presence of a conserved 'exportome' in the genus Plasmodium has important implications for our understanding of both common mechanisms and species-specific differences in host-parasite interactions, and may be crucial in developing novel antimalarial drugs to this infectious disease.
Figures





References
-
- Waller KL, Cooke BM, Nunomura W, Mohandas N, Coppel RL. Mapping the binding domains involved in the interaction between the Plasmodium falciparum knob-associated histidine-rich protein (KAHRP) and the cytoadherence ligand P. falciparum erythrocyte membrane protein 1 (PfEMP1). J Biol Chem. 1999;274:23808–23813. doi: 10.1074/jbc.274.34.23808. - DOI - PubMed
-
- Freitas-Junior LH, Hernandez-Rivas R, Ralph SA, Montiel-Condado D, Ruvalcaba-Salazar OK, Rojas-Meza AP, Mancio-Silva L, Leal-Silvestre RJ, Gontijo AM, Shorte S, Scherf A. Telomeric heterochromatin propagation and histone acetylation control mutually exclusive expression of antigenic variation genes in malaria parasites. Cell. 2005;121:25–36. doi: 10.1016/j.cell.2005.01.037. - DOI - PubMed
-
- Duraisingh MT, Voss TS, Marty AJ, Duffy MF, Good RT, Thompson JK, Freitas-Junior LH, Scherf A, Crabb BS, Cowman AF. Heterochromatin silencing and locus repositioning linked to regulation of virulence genes in Plasmodium falciparum. Cell. 2005;121:13–24. doi: 10.1016/j.cell.2005.01.036. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

