Expression of the inflammatory chemokines CCL5, CCL3 and CXCL10 in juvenile idiopathic arthritis, and demonstration of CCL5 production by an atypical subset of CD8+ T cells
- PMID: 16507178
- PMCID: PMC1526593
- DOI: 10.1186/ar1913
Expression of the inflammatory chemokines CCL5, CCL3 and CXCL10 in juvenile idiopathic arthritis, and demonstration of CCL5 production by an atypical subset of CD8+ T cells
Abstract
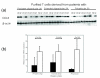
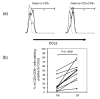
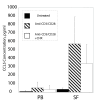
This study focuses upon three chemokines, namely CCL5, CXCL10 and CCL3, which are potential novel therapeutic targets in arthritis. The aim of the study was to analyse the expression and production of these three chemokines within the joints of children with juvenile idiopathic arthritis (JIA) of the oligoarticular and polyarticular subtypes. All three of these chemokines are highly expressed at the level of mRNA, with the most significant increase in mRNA levels being demonstrated for CCL5 when compared with matched peripheral blood samples and controls. We show that high levels of all three chemokines are present in synovial fluid of children with JIA. We investigate the major source of CCL5 from inflammatory synovial cells, which we show to be CD8+ T cells. This CD8+ synovial T cell population has an unexpected phenotype that has not been described previously, being CCR7- yet predominantly CD28+ and CD45RA-. These cells contain high levels of stored intracellular CCL5, and rapid release of CCL5 takes place on T cell stimulation, without requiring new protein synthesis. In addition, we demonstrate that CCL5 is present in synovial biopsies from these patients, in particular on the endothelium of small and medium sized vessels. We believe this to be the first in depth analysis of these mediators of inflammation in JIA.
Figures



References
-
- Bywaters EG. Pathologic aspects of juvenile chronic polyarthritis. Arthritis Rheum. 1977;20:271–276. - PubMed
-
- Butcher EC, Scollay RG, Weissman IL. Organ specificity of lymphocyte migration: mediation by highly selective lymphocyte interaction with organ-specific determinants on high endothelial venules. Eur J Immunol. 1980;10:556–561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

