Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure
- PMID: 16507465
- PMCID: PMC1392236
- DOI: 10.1289/ehp.8413
Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure
Abstract
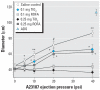
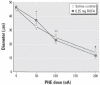
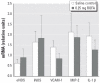
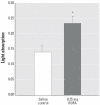
The epidemiologic association between pulmonary exposure to ambient particulate matter (PM) and cardiovascular dysfunction is well known, but the systemic mechanisms that drive this effect remain unclear. We have previously shown that acute pulmonary exposure to PM impairs or abolishes endothelium-dependent arteriolar dilation in the rat spinotrapezius muscle. The purpose of this study was to further characterize the effect of pulmonary PM exposure on systemic microvascular function and to identify local inflammatory events that may contribute to these effects. Rats were intratracheally instilled with residual oil fly ash (ROFA) or titanium dioxide at 0.1 or 0.25 mg/rat 24 hr before measurement of pulmonary and systemic microvascular responses. In vivo microscopy of the spinotrapezius muscle was used to study systemic arteriolar responses to intraluminal infusion of the Ca2+ ionophore A23187 or iontophoretic abluminal application of the adrenergic agonist phenylephrine (PHE). Leukocyte rolling and adhesion were quantified in venules paired with the studied arterioles. Histologic techniques were used to assess pulmonary inflammation, characterize the adherence of leukocytes to systemic venules, verify the presence of myeloperoxidase (MPO) in the systemic microvascular wall, and quantify systemic microvascular oxidative stress. In the lungs of rats exposed to ROFA or TiO2, changes in some bronchoalveolar lavage markers of inflammation were noted, but an indication of cellular damage was not found. In rats exposed to 0.1 mg ROFA, focal alveolitis was evident, particularly at sites of particle deposition. Exposure to either ROFA or TiO2 caused a dose-dependent impairment of endothelium-dependent arteriolar dilation. However, exposure to these particles did not affect microvascular constriction in response to PHE. ROFA and TiO2 exposure significantly increased leukocyte rolling and adhesion in paired venules, and these cells were positively identified as polymorphonuclear leukocytes (PMNLs). In ROFA- and TiO2-exposed rats, MPO was found in PMNLs adhering to the systemic microvascular wall. Evidence suggests that some of this MPO had been deposited in the microvascular wall. There was also evidence for oxidative stress in the microvascular wall. These results indicate that after PM exposure, the impairment of endothelium-dependent dilation in the systemic microcirculation coincides with PMNL adhesion, MPO deposition, and local oxidative stress. Collectively, these microvascular observations are consistent with events that contribute to the disruption of the control of peripheral resistance and/or cardiac dysfunction associated with PM exposure.
Figures




References
-
- Antonini JM, Roberts JR, Jernigan MR, Yang HM, Ma JY, Clarke RW. Residual oil fly ash increases the susceptibility to infection and severely damages the lungs after pulmonary challenge with a bacterial pathogen. Toxicol Sci. 2002;70:110–119. - PubMed
-
- Bagate K, Meiring JJ, Cassee FR, Borm PJ. The effect of particulate matter on resistance and conductance vessels in the rat. Inhal Toxicol. 2004;16:431–436. - PubMed
-
- Brain JD, Knudson DE, Sorokin SP, Davis MA. Pulmonary distribution of particles given by intratracheal instillation or by aerosol inhalation. Environ Res. 1976;11:13–33. - PubMed
-
- Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–1536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
