Fontolizumab, a humanised anti-interferon gamma antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn's disease
- PMID: 16507585
- PMCID: PMC1856291
- DOI: 10.1136/gut.2005.079392
Fontolizumab, a humanised anti-interferon gamma antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn's disease
Abstract
Introduction: Interferon gamma is a potent proinflammatory cytokine implicated in the inflammation of Crohn's disease (CD). We evaluated the safety and efficacy of fontolizumab, a humanised anti-interferon gamma antibody, in patients with moderate to severe CD.
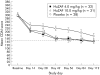
Methods: A total of 133 patients with Crohn's disease activity index (CDAI) scores between 250 and 450, inclusive, were randomised to receive placebo or fontolizumab 4 or 10 mg/kg. Forty two patients received one dose and 91 patients received two doses on days 0 and 28. Investigators and patients were unaware of assignment. Study end points were safety, clinical response (decrease in CDAI of 100 points or more), and remission (CDAI < or =150).
Results: There was no statistically significant difference in the primary end point of the study (clinical response) between the fontolizumab and placebo groups after a single dose at day 28. However, patients receiving two doses of fontolizumab demonstrated doubling in response rate at day 56 compared with placebo: 32% (9/28) versus 69% (22/32, p = 0.02) and 67% (21/31, p = 0.03) for the placebo, and 4 and 10 mg/kg fontolizumab groups, respectively. Stratification according to elevated baseline C reactive protein levels resulted in a decreased placebo response and pronounced differences in clinical benefit. Two grade 3 adverse events were reported and were considered to be related to CD. One death (during sleep) and one serious adverse event (an elective hospitalisation) occurred, both considered unrelated.
Conclusion: Treating active CD with fontolizumab was well tolerated and resulted in increased rates of clinical response and remission compared with placebo.
Comment in
-
Interfering with interferons in inflammatory bowel disease.Gut. 2006 Aug;55(8):1071-3. doi: 10.1136/gut.2005.090134. Gut. 2006. PMID: 16849343 Free PMC article. Review.
References
-
- Rennick D M, Fort M M, Davidson N J. Studies with IL‐10−/− mice: an overview. J Leukoc Biol 199761389–396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials