The role of KAHRP domains in knob formation and cytoadherence of P falciparum-infected human erythrocytes
- PMID: 16507777
- PMCID: PMC1895844
- DOI: 10.1182/blood-2005-11-4624
The role of KAHRP domains in knob formation and cytoadherence of P falciparum-infected human erythrocytes
Abstract
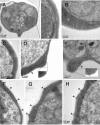
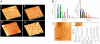
Surface protrusions of Plasmodium falciparum-infected erythrocytes, called knobs, display focal aggregates of P falciparum erythrocyte membrane protein 1 (PfEMP1), the adhesion ligand binding endothelial-cell receptors. The resulting sequestration of infected erythrocytes in tissues represents an important factor in the course of fatalities in patients with malaria. The main component of knobs is the knob-associated histidine-rich protein (KAHRP), and it contributes to altered mechanical properties of parasite-infected erythrocytes. The role of KAHRP domains in these processes is still elusive. We generated stable transgenic P falciparum-infected erythrocytes expressing mutant versions of KAHRP. Using atomic force and electron microscopy we show that the C-terminal repeat region is critical for the formation of functional knobs. Elasticity of the membrane differs dramatically between cells with different KAHRP mutations. We propose that the 5' repeat region of KAHRP is important in cross-linking to the host-cell cytoskeleton and this is required for knob protrusion and efficient adhesion under physiologic flow conditions.
Figures
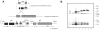


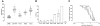

Similar articles
-
Plasmodium falciparum erythrocyte membrane protein 1 is anchored to the actin-spectrin junction and knob-associated histidine-rich protein in the erythrocyte skeleton.Mol Biochem Parasitol. 2000 May;108(2):237-47. doi: 10.1016/s0166-6851(00)00227-9. Mol Biochem Parasitol. 2000. PMID: 10838226
-
Dynamic association of PfEMP1 and KAHRP in knobs mediates cytoadherence during Plasmodium invasion.Sci Rep. 2015 Mar 2;5:8617. doi: 10.1038/srep08617. Sci Rep. 2015. PMID: 25726759 Free PMC article.
-
A spiral scaffold underlies cytoadherent knobs in Plasmodium falciparum-infected erythrocytes.Blood. 2016 Jan 21;127(3):343-51. doi: 10.1182/blood-2015-10-674002. Epub 2015 Dec 4. Blood. 2016. PMID: 26637786 Free PMC article.
-
Knobs, knob proteins and cytoadherence in falciparum malaria.Int J Biochem. 1991;23(9):775-89. doi: 10.1016/0020-711x(91)90061-q. Int J Biochem. 1991. PMID: 1773882 Review.
-
Knobs, Adhesion, and Severe Falciparum Malaria.Trop Med Infect Dis. 2023 Jul 4;8(7):353. doi: 10.3390/tropicalmed8070353. Trop Med Infect Dis. 2023. PMID: 37505649 Free PMC article. Review.
Cited by
-
Ultrastructural alterations in Plasmodium falciparum induced by chalcone derivatives.BMC Res Notes. 2020 Jun 15;13(1):290. doi: 10.1186/s13104-020-05132-z. BMC Res Notes. 2020. PMID: 32539868 Free PMC article.
-
Clag9 is not essential for PfEMP1 surface expression in non-cytoadherent Plasmodium falciparum parasites with a chromosome 9 deletion.PLoS One. 2011;6(12):e29039. doi: 10.1371/journal.pone.0029039. Epub 2011 Dec 19. PLoS One. 2011. PMID: 22205992 Free PMC article.
-
Tetracysteine-based fluorescent tags to study protein localization and trafficking in Plasmodium falciparum-infected erythrocytes.PLoS One. 2011;6(8):e22975. doi: 10.1371/journal.pone.0022975. Epub 2011 Aug 10. PLoS One. 2011. PMID: 21860664 Free PMC article.
-
Heat Shock Proteins as Targets for Novel Antimalarial Drug Discovery.Adv Exp Med Biol. 2021;1340:205-236. doi: 10.1007/978-3-030-78397-6_9. Adv Exp Med Biol. 2021. PMID: 34569027
-
Gene copy number variation in natural populations of Plasmodium falciparum in Eastern Africa.BMC Genomics. 2018 May 21;19(1):372. doi: 10.1186/s12864-018-4689-7. BMC Genomics. 2018. PMID: 29783949 Free PMC article.
References
-
- Cooke BM, Mohandas N, Coppel RL. Malaria and the red blood cell membrane. Semin Hematol. 2004;41: 173-188. - PubMed
-
- Baruch DI, Rogerson SJ, Cooke BM. Asexual blood stages of malaria antigens: cytoadherence. Chem Immunol. 2002;80: 144-162. - PubMed
-
- Su XZ, Heatwole VM, Wertheimer SP, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82: 89-100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

