Cell-cycle markers in a transgenic mouse model of human tauopathy: increased levels of cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1
- PMID: 16507903
- PMCID: PMC1606514
- DOI: 10.2353/ajpath.2006.050540
Cell-cycle markers in a transgenic mouse model of human tauopathy: increased levels of cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1
Abstract
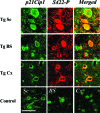
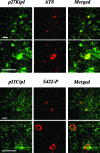
Recent evidence has suggested that an abnormal reactivation of the cell cycle may precede and cause the hyperphosphorylation and filament formation of tau protein in Alzheimer's disease and other tauopathies. Here we have analyzed the expression and/or activation of proteins involved in cell-cycle progression in the brain and spinal cord of mice transgenic for mutant human P301S tau protein. This mouse line recapitulates the essential molecular and cellular features of the human tauopathies, including hyperphosphorylation and filament formation of tau protein. None of the activators and co-activators of the cell cycle tested were overexpressed or activated in 5-month-old transgenic mice when compared to controls. By contrast, the levels of cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1 were increased in brain and spinal cord of transgenic mice. Both inhibitors accumulated in the cytoplasm of nerve cells, the majority of which contained inclusions made of hyperphosphorylated tau protein. A similar staining pattern for p21Cip1 and p27Kip1 was also present in the frontal cortex from a case of FTDP-17 with the P301L tau mutation. Thus, reactivation of the cell cycle was not involved in tau hyperphos-phorylation and filament formation, consistent with expression of p21Cip1 and p27Kip1 in tangle-bearing nerve cells.
Figures


Similar articles
-
Analysis of tau phosphorylation and truncation in a mouse model of human tauopathy.Am J Pathol. 2008 Jan;172(1):123-31. doi: 10.2353/ajpath.2008.070627. Epub 2007 Dec 13. Am J Pathol. 2008. PMID: 18079436 Free PMC article.
-
TGF-beta mediated G1 arrest in a human melanoma cell line lacking p15INK4B: evidence for cooperation between p21Cip1/WAF1 and p27Kip1.Oncogene. 1996 Dec 5;13(11):2447-57. Oncogene. 1996. PMID: 8957087
-
RANKL coordinates cell cycle withdrawal and differentiation in osteoclasts through the cyclin-dependent kinase inhibitors p27KIP1 and p21CIP1.J Bone Miner Res. 2004 Aug;19(8):1339-48. doi: 10.1359/JBMR.040321. Epub 2004 Mar 29. J Bone Miner Res. 2004. PMID: 15231022
-
Analysis of tauopathies with transgenic mice.Trends Mol Med. 2001 Oct;7(10):467-70. doi: 10.1016/s1471-4914(01)02123-2. Trends Mol Med. 2001. PMID: 11597522 Review.
-
Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer's disease and tauopathies.Curr Alzheimer Res. 2005 Jan;2(1):3-18. doi: 10.2174/1567205052772713. Curr Alzheimer Res. 2005. PMID: 15977985 Review.
Cited by
-
Role of tumor suppressor molecules in genomic perturbations and damaged DNA repair involved in the pathogenesis of cancer and neurodegeneration (Review).Biomed Rep. 2020 Sep;13(3):10. doi: 10.3892/br.2020.1317. Epub 2020 Jun 17. Biomed Rep. 2020. PMID: 32765849 Free PMC article. Review.
-
Aberrant Neuronal Cell Cycle Re-Entry: The Pathological Confluence of Alzheimer's Disease and Brain Insulin Resistance, and Its Relation to Cancer.J Alzheimers Dis. 2019;67(1):1-11. doi: 10.3233/JAD-180874. J Alzheimers Dis. 2019. PMID: 30452418 Free PMC article. Review.
-
Transcriptome analysis of a tau overexpression model in rats implicates an early pro-inflammatory response.Exp Neurol. 2010 Jul;224(1):197-206. doi: 10.1016/j.expneurol.2010.03.011. Epub 2010 Mar 24. Exp Neurol. 2010. PMID: 20346943 Free PMC article.
-
Ageing, Cellular Senescence and Neurodegenerative Disease.Int J Mol Sci. 2018 Sep 27;19(10):2937. doi: 10.3390/ijms19102937. Int J Mol Sci. 2018. PMID: 30261683 Free PMC article. Review.
-
Neurogenesis and cell cycle-reactivated neuronal death during pathogenic tau aggregation.Genes Brain Behav. 2008 Feb;7 Suppl 1(1):92-100. doi: 10.1111/j.1601-183X.2007.00377.x. Genes Brain Behav. 2008. PMID: 18184373 Free PMC article.
References
-
- Goedert M, Spillantini MG, Davies SW. Filamentous nerve cell inclusions in neurodegenerative diseases. Curr Opin Neurobiol. 1998;8:619–632. - PubMed
-
- Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Rev. 2000;33:95–130. - PubMed
-
- Lee VMY, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. - PubMed
-
- Poorkaj P, Bird TD, Wijsman E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. - PubMed
-
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski JQ, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JBJ, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5′-splice-site-mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

