Plectin regulates the organization of glial fibrillary acidic protein in Alexander disease
- PMID: 16507904
- PMCID: PMC1606531
- DOI: 10.2353/ajpath.2006.051028
Plectin regulates the organization of glial fibrillary acidic protein in Alexander disease
Abstract
Alexander disease (AxD) is a rare but fatal neurological disorder caused by mutations in the astrocyte-specific intermediate filament protein glial fibrillary acidic protein (GFAP). Histologically, AxD is characterized by cytoplasmic inclusion bodies called Rosenthal fibers (RFs), which contain GFAP, small heat shock proteins, and other undefined components. Here, we describe the expression of the cytoskeletal linker protein plectin in the AxD brain. RFs displayed positive immunostaining for plectin and GFAP, both of which were increased in the AxD brain. Co-localization, co-immunoprecipitation, and in vitro overlay analyses demonstrated direct interaction of plectin and GFAP. GFAP with the most common AxD mutation, R239C (RC GFAP), mainly formed abnormal aggregates in human primary astrocytes and murine plectin-deficient fibroblasts. Transient transfection of full-length plectin cDNA converted these aggregates to thin filaments, which exhibited diffuse cytoplasmic distribution. Compared to wild-type GFAP expression, RC GFAP expression lowered plectin levels in astrocytoma-derived stable transfectants and plectin-positive fibroblasts. A much higher proportion of total GFAP was found in the Triton X-insoluble fraction of plectin-deficient fibroblasts than in wild-type fibroblasts. Taken together, our results suggest that insufficient amounts of plectin, due to RC GFAP expression, promote GFAP aggregation and RF formation in AxD.
Figures


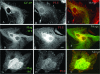






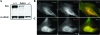
Similar articles
-
Synemin is expressed in reactive astrocytes and Rosenthal fibers in Alexander disease.APMIS. 2014 Jan;122(1):76-80. doi: 10.1111/apm.12088. Epub 2013 Apr 18. APMIS. 2014. PMID: 23594359
-
The origin of Rosenthal fibers and their contributions to astrocyte pathology in Alexander disease.Acta Neuropathol Commun. 2017 Mar 31;5(1):27. doi: 10.1186/s40478-017-0425-9. Acta Neuropathol Commun. 2017. PMID: 28359321 Free PMC article.
-
Dynamics of mutated GFAP aggregates revealed by real-time imaging of an astrocyte model of Alexander disease.Exp Cell Res. 2007 Aug 1;313(13):2766-79. doi: 10.1016/j.yexcr.2007.04.035. Epub 2007 May 24. Exp Cell Res. 2007. PMID: 17604020
-
Clinical aspects and pathology of Alexander disease, and morphological and functional alteration of astrocytes induced by GFAP mutation.Neuropathology. 2012 Aug;32(4):440-6. doi: 10.1111/j.1440-1789.2011.01268.x. Epub 2011 Nov 28. Neuropathology. 2012. PMID: 22118268 Review.
-
[Glial fibrillary acidic protein: the component of intermediate filaments in the vertebrate brain astrocytes].Zh Evol Biokhim Fiziol. 2015 Jan-Feb;51(1):3-10. Zh Evol Biokhim Fiziol. 2015. PMID: 25859599 Review. Russian.
Cited by
-
Accumulation of glial fibrillary acidic protein and histone H4 in brain storage bodies of Tibetan terriers with hereditary neuronal ceroid lipofuscinosis.J Inherit Metab Dis. 2007 Nov;30(6):952-63. doi: 10.1007/s10545-007-0683-y. Epub 2007 Nov 15. J Inherit Metab Dis. 2007. PMID: 18004671
-
Fangchinoline induces autophagic cell death via p53/sestrin2/AMPK signalling in human hepatocellular carcinoma cells.Br J Pharmacol. 2011 Sep;164(2b):731-42. doi: 10.1111/j.1476-5381.2011.01349.x. Br J Pharmacol. 2011. PMID: 21418191 Free PMC article.
-
Plectin-controlled keratin cytoarchitecture affects MAP kinases involved in cellular stress response and migration.J Cell Biol. 2006 Aug 14;174(4):557-68. doi: 10.1083/jcb.200605172. J Cell Biol. 2006. PMID: 16908671 Free PMC article.
-
Dysfunctions of neuronal and glial intermediate filaments in disease.J Clin Invest. 2009 Jul;119(7):1814-24. doi: 10.1172/JCI38003. Epub 2009 Jul 1. J Clin Invest. 2009. PMID: 19587456 Free PMC article. Review.
-
Phenotypic conversions of "protoplasmic" to "reactive" astrocytes in Alexander disease.J Neurosci. 2013 Apr 24;33(17):7439-50. doi: 10.1523/JNEUROSCI.4506-12.2013. J Neurosci. 2013. PMID: 23616550 Free PMC article.
References
-
- Messing A, Goldman JE, Johnson AB, Brenner M. Alexander disease: new insights from genetics. J Neuropathol Exp Neurol. 2001;60:563–573. - PubMed
-
- Brenner M, Johnson AB, Boespflug-Tanguy O, Rodriguez D, Goldman JE, Messing A. Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat Genet. 2001;27:117–120. - PubMed
-
- Rodriguez D, Gauthier F, Bertini E, Bugiani M, Brenner M, N′Guyen S, Goizet C, Gelot A, Surtees R, Pedespan JM, Hernandorena X, Troncoso M, Uziel G, Messing A, Ponsot G, Pham-Dinh D, Dautigny A, Boespflug-Tanguy O. Infantile Alexander disease: spectrum of GFAP mutations and genotype-phenotype correlation. Am J Hum Genet. 2001;69:1134–1140. - PMC - PubMed
-
- Wiche G, Herrmann H, Leichtfried F, Pytela R. Plectin: a high-molecular-weight cytoskeletal polypeptide component that copurifies with intermediate filaments of the vimentin type. Cold Spring Harb Symp Quant Biol. 1982;46:475–482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

