Transmission of amyloidosis in offspring of mice with AApoAII amyloidosis
- PMID: 16507905
- PMCID: PMC1606535
- DOI: 10.2353/ajpath.2006.050350
Transmission of amyloidosis in offspring of mice with AApoAII amyloidosis
Abstract
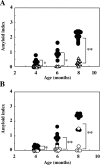
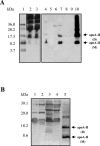
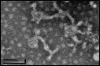
Pre-existing amyloid fibrils can induce further polymerization of endogenous precursor proteins in vivo. Thus, transmission of amyloid fibrils (AApoAII) may induce a conformational change in endogenous apolipoprotein A-II and accelerate amyloid deposition in mouse senile amyloidosis. To characterize transmissibility, we examined amyloidosis in the offspring of AApoAII-injected mother mice that possessed the amyloidogenic Apoa2(c) allele of the apolipoprotein A-II gene. At 4 months of age, amyloid deposits were detected in the intestines of offspring born from and nursed by amyloid fibril-injected mothers, with intensity of deposition increasing thereafter. No amyloid deposits were detected in the offspring of noninjected control mothers. Accelerated amyloidosis was also observed in offspring born from mothers without injection but nursed by amyloid fibril-injected mothers. However, this was not observed in offspring born from amyloid fibril-injected mothers but nursed by control mothers. This fostering excluded vertical transmission through the placenta, suggesting the presence of factors that accelerate amyloidosis during the nursing period. In addition, milk obtained from amyloid fibril-injected mothers induced AApoAII amyloidosis in young mice, and transmission electron microscopy detected noodle-like amyloid fibrils in milk of amyloid fibril-injected mothers. These results provide important insight into the etiology and pathogenesis of amyloid diseases.
Figures


References
-
- Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900–904. - PubMed
-
- Glenner GG. Amyloid deposits and amyloidosis: the beta-fibrilloses. N Engl J Med. 1980;302:1283–1292. - PubMed
-
- Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–596. - PubMed
-
- Jarrett JT, Lansbury PT., Jr Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73:1055–1058. - PubMed
-
- Rochet JC, Lansbury PT., Jr Amyloid fibrillogenesis: themes and variations. Curr Opin Struct Biol. 2000;10:60–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

