Identification of differential protein expression associated with development of unstable human carotid plaques
- PMID: 16507914
- PMCID: PMC1606543
- DOI: 10.2353/ajpath.2006.050471
Identification of differential protein expression associated with development of unstable human carotid plaques
Abstract
Rupture-prone unstable arterial plaques develop concomitantly with the appearance of intraplaque hemorrhage and tissue ulceration, in association with deregulation of smooth muscle cell mitogenesis and leakage of newly formed blood vessels. Using microarray technology, we have identified novel protein deregulation associated with unstable carotid plaque regions. Overexpression of proapoptotic proteins caspase-9 and TRAF4 was seen in endothelial cells and smooth muscle cells from unstable hemorrhagic and ulcerated plaque regions. Topoisomerase-II-alpha (TOPO-II-alpha), which is associated with DNA repair mechanisms, was also overexpressed by these cells. Cell signaling molecules c-src, G-protein-coupled receptor kinase-interacting protein (GIT1), and c-jun N-terminal kinase (JNK) were up-regulated in endothelial cells from the same areas, whereas an increase in expression of junctional adhesion molecule-1 (JAM-1) in blood vessels and infiltrating macrophages from inflammatory regions might form part of a leukocyte rolling response, increasing the plaque volume. Grb2-like adaptor protein (Gads), responsible for differentiation of monocytes into macrophages, was expressed by macrophages from unstable plaques, suggesting a potential mechanism through which increased scavenging could occur in rupture-prone areas. We conclude that modulation of novel cell signaling intermediates, such as those described here, could be useful in the therapy of angiogenesis and apoptosis, designed to reduce unstable plaque formation.
Figures
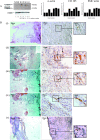



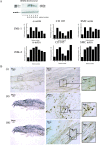
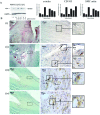


References
-
- Hennerici MG. The unstable plaque. Cerebrovasc Dis. 2004;17:17–22. - PubMed
-
- Moulton KS. Plaque angiogenesis: its function and regulation. Cold Spring Harbor Symp Quant Biol. 2002;11:471–482. - PubMed
-
- DeGraba TJ, Siren AL, Penix L, McCarron RM, Hargraves R, Sood S, Pettigrew KD, Hallenbeck JM. Increased endothelial expression of intercellular adhesion molecule-1 in symptomatic versus asymptomatic human carotid atherosclerotic plaque. Stroke. 1998;29:1405–1410. - PubMed
-
- Loftus IM, Naylor AR, Goodall S, Crowther M, Jones L, Bell PR, Thompson MM. Increased matrix metalloproteinase-9 activity in unstable carotid plaques. A potential role in acute plaque disruption. Stroke. 2000;31:40–47. - PubMed
-
- Mallat Z, Corbaz A, Scoazec A, Besnard S, Leseche G, Chvatchko Y, Tedgui A. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. 2001;104:1598–1603. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

