Renewal of mural thrombus releases plasma markers and is involved in aortic abdominal aneurysm evolution
- PMID: 16507915
- PMCID: PMC1606522
- DOI: 10.2353/ajpath.2006.050868
Renewal of mural thrombus releases plasma markers and is involved in aortic abdominal aneurysm evolution
Abstract
Human abdominal aortic aneurysm (AAA) expansion has been linked to the presence of a mural thrombus. Here we explored the mechanism of the continual luminal renewal of this thrombus and its ability to release biological markers potentially detectable in plasma. We also explored the ability of platelet inhibition to pacify the thrombus and to limit aneurysm progression in an experimental model. Blood samples and mural thrombi were collected in 20 AAA patients. In parallel, segments of sodium dodecyl sulfate-decellularized guinea pig aorta were xenografted onto the abdominal aorta of 30 rats to induce aneurysms. Fifteen rats received abciximab treatment and fifteen received irrelevant immunoglobulins. Procoagulant activity and platelet activation markers (microparticles, sP-selectin, sGPV, sCD40L) were increased threefold to fivefold in eluates from the luminal thrombus layer compared to other layers. All these markers were increased twofold to fivefold in patients' plasma compared to matched controls (P < 0.005). In the rat model, abciximab reduced both thrombus area and aneurysmal enlargement (P < 0.05). Platelet aggregation is probably responsible for the renewal of the thrombus in AAA. The luminal thrombus released markers of platelet activation that could easily be detected in plasma. Platelet inhibition limited aortic aneurysm expansion in a rat model, providing new therapeutic perspectives in the prevention of AAA enlargement.
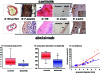
Figures



References
-
- Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation. 2004;110:16–21. - PubMed
-
- Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365:1577–1589. - PubMed
-
- Adolph R, Vorp DA, Steed DL, Webster MW, Kameneva MV, Watkins SC. Cellular content and permeability of intraluminal thrombus in abdominal aortic aneurysm. J Vasc Surg. 1997;25:916–926. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

