Histone deacetylase inhibitors induce VHL and ubiquitin-independent proteasomal degradation of hypoxia-inducible factor 1alpha
- PMID: 16507982
- PMCID: PMC1430285
- DOI: 10.1128/MCB.26.6.2019-2028.2006
Histone deacetylase inhibitors induce VHL and ubiquitin-independent proteasomal degradation of hypoxia-inducible factor 1alpha
Abstract
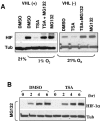
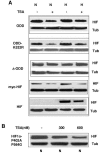
Adaptation to hypoxic microenvironment is critical for tumor survival and metastatic spread. Hypoxia-inducible factor 1alpha (HIF-1alpha) plays a key role in this adaptation by stimulating the production of proangiogenic factors and inducing enzymes necessary for anaerobic metabolism. Histone deacetylase inhibitors (HDACIs) produce a marked inhibition of HIF-1alpha expression and are currently in clinical trials partly based on their potent antiangiogenic effects. Although it has been postulated that HDACIs affect HIF-1alpha expression by enhancing its interactions with VHL (von Hippel Lindau), thus promoting its ubiquitination and degradation, the actual mechanisms by which HDACIs decrease HIF-1alpha levels are not clear. Here, we present data indicating that HDACIs induce the proteasomal degradation of HIF-1alpha by a mechanism that is independent of VHL and p53 and does not require the ubiquitin system. This degradation pathway involves the enhanced interaction of HIF-1alpha with HSP70 and is secondary to a disruption of the HSP70/HSP90 axis function that appears mediated by the activity of HDAC-6.
Figures






Similar articles
-
JNK1 mediates degradation HIF-1alpha by a VHL-independent mechanism that involves the chaperones Hsp90/Hsp70.Cancer Res. 2010 Jan 15;70(2):813-23. doi: 10.1158/0008-5472.CAN-09-0448. Epub 2010 Jan 12. Cancer Res. 2010. PMID: 20068160 Free PMC article.
-
PI3K/Akt is required for heat shock proteins to protect hypoxia-inducible factor 1alpha from pVHL-independent degradation.J Biol Chem. 2004 Apr 2;279(14):13506-13. doi: 10.1074/jbc.M310164200. Epub 2004 Jan 15. J Biol Chem. 2004. PMID: 14726529
-
AK-1, a SIRT2 inhibitor, destabilizes HIF-1α and diminishes its transcriptional activity during hypoxia.Cancer Lett. 2016 Apr 1;373(1):138-145. doi: 10.1016/j.canlet.2016.01.031. Epub 2016 Jan 22. Cancer Lett. 2016. PMID: 26808575
-
Melatonin and the von Hippel-Lindau/HIF-1 oxygen sensing mechanism: A review.Biochim Biophys Acta. 2016 Apr;1865(2):176-83. doi: 10.1016/j.bbcan.2016.02.004. Epub 2016 Feb 17. Biochim Biophys Acta. 2016. PMID: 26899267 Review.
-
RACK1 vs. HSP90: competition for HIF-1 alpha degradation vs. stabilization.Cell Cycle. 2007 Mar 15;6(6):656-9. doi: 10.4161/cc.6.6.3981. Epub 2007 Mar 7. Cell Cycle. 2007. PMID: 17361105 Review.
Cited by
-
Inhibition of histone deacetylase impacts cancer stem cells and induces epithelial-mesenchyme transition of head and neck cancer.PLoS One. 2013;8(3):e58672. doi: 10.1371/journal.pone.0058672. Epub 2013 Mar 20. PLoS One. 2013. PMID: 23527004 Free PMC article.
-
HDACs and HDAC Inhibitors in Cancer Development and Therapy.Cold Spring Harb Perspect Med. 2016 Oct 3;6(10):a026831. doi: 10.1101/cshperspect.a026831. Cold Spring Harb Perspect Med. 2016. PMID: 27599530 Free PMC article. Review.
-
Reduced HIF-1α Stability Induced by 6-Gingerol Inhibits Lung Cancer Growth through the Induction of Cell Death.Molecules. 2022 Mar 24;27(7):2106. doi: 10.3390/molecules27072106. Molecules. 2022. PMID: 35408505 Free PMC article.
-
Targeting HIF-1α Function in Cancer through the Chaperone Action of NQO1: Implications of Genetic Diversity of NQO1.J Pers Med. 2022 May 5;12(5):747. doi: 10.3390/jpm12050747. J Pers Med. 2022. PMID: 35629169 Free PMC article. Review.
-
Modulation of hypoxia-inducible factors (HIF) from an integrative pharmacological perspective.Cell Mol Life Sci. 2012 Feb;69(4):519-34. doi: 10.1007/s00018-011-0813-4. Epub 2011 Oct 8. Cell Mol Life Sci. 2012. PMID: 21984597 Free PMC article. Review.
References
-
- Bali, P., M. Pranpat, J. Bradner, M. Balasis, W. Fiskus, F. Guo, K. Rocha, S. Kumaraswamy, S. Boyapalle, P. Atadja, E. Seto, and K. Bhalla. 2005. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J. Biol. Chem. 280:26729-26734. - PubMed
-
- Bilton, R., N. Mazure, E. Trottier, M. Hattab, M. A. Dery, D. E. Richard, J. Pouyssegur, and M. C. Brahimi-Horn. 2005. Arrest-defective-1 protein, an acetyltransferase, does not alter stability of hypoxia-inducible factor (HIF)-1α and is not induced by hypoxia or HIF. J. Biol. Chem. 280:31132-31140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
