p38 mitogen-activated protein kinase mediates the Fas-induced mitochondrial death pathway in CD8+ T cells
- PMID: 16507991
- PMCID: PMC1430304
- DOI: 10.1128/MCB.26.6.2118-2129.2006
p38 mitogen-activated protein kinase mediates the Fas-induced mitochondrial death pathway in CD8+ T cells
Abstract
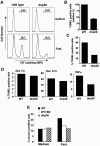
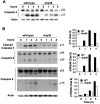
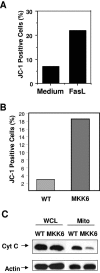
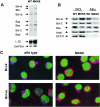
The p38 mitogen-activated protein kinase (MAPK) signaling pathway can be activated by a variety of stress stimuli such as UV radiation and osmotic stress. The regulation and role of this pathway in death receptor-induced apoptosis remain unclear and may depend on the specific death receptor and cell type. Here we show that binding of Fas ligand to Fas activates p38 MAPK in CD8+ T cells and that activation of this pathway is required for Fas-mediated CD8+ T-cell death. Active p38 MAPK phosphorylates Bcl-xL and Bcl-2 and prevents the accumulation of these antiapoptotic molecules within the mitochondria. Consequently, a loss of mitochondrial membrane potential and the release of cytochrome c lead to the activation of caspase 9 and, subsequently, caspase 3. Therefore, the activation of p38 MAPK is a critical link between Fas and the mitochondrial death pathway and is required for the Fas-induced apoptosis of CD8+ T cells.
Figures




References
-
- Braz, J. C., O. F. Bueno, Q. Liang, B. J. Wilkins, Y.-S. Dai, S. Parsons, J. Braunwart, B. J. Glascock, R. Klevitsky, T. F. Kimball, T. E. Hewett, and J. D. Molkentin. 2003. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J. Clin. Investig. 111:1475-1486. - PMC - PubMed
-
- Brenner, B., U. Koppenhoefer, C. Weinstock, O. Linderkamp, F. Lang, and E. Gulbins. 1997. Fas- or ceramide-induced apoptosis is mediated by a Rac1-regulated activation of Jun N-terminal kinase/p38 kinases and GADD153. J. Biol. Chem. 272:22173-22181. - PubMed
-
- Cahill, M. A., M. E. Peter, F. C. Kischkel, A. M. Chinnaiyan, V. M. Dixit, P. H. Krammer, and A. Nordheim. 1996. CD95 (APO-1/Fas) induces activation of SAP kinases downstream of ICE-like proteases. Oncogene 13:2087-2096. - PubMed
-
- Cohen, P. L., and R. A. Eisenberg. 1991. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu. Rev. Immunol. 9:243-269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
