A family knockout of all four Drosophila metallothioneins reveals a central role in copper homeostasis and detoxification
- PMID: 16508004
- PMCID: PMC1430275
- DOI: 10.1128/MCB.26.6.2286-2296.2006
A family knockout of all four Drosophila metallothioneins reveals a central role in copper homeostasis and detoxification
Abstract
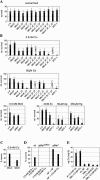
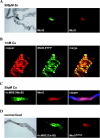
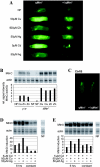
Metallothioneins are ubiquitous, small, cysteine-rich proteins with the ability to bind heavy metals. In spite of their biochemical characterization, their in vivo function remains elusive. Here, we report the generation of a metallothionein gene family knockout in Drosophila melanogaster by targeted disruption of all four genes (MtnA to -D). These flies are viable if raised in standard laboratory food. During development, however, they are highly sensitive to copper, cadmium, and (to a lesser extent) zinc load. Metallothionein expression is particularly important for male viability; while copper load during development affects males and females equally, adult males lacking metallothioneins display a severely reduced life span, possibly due to copper-mediated oxidative stress. Using various reporter gene constructs, we find that different metallothioneins are expressed with virtually the same tissue specificity in larvae, notably in the intestinal tract at sites of metal accumulation, including the midgut's "copper cells." The same expression pattern is observed with a synthetic minipromoter consisting only of four tandem metal response elements. From these and other experiments, we conclude that tissue specificity of metallothionein expression is a consequence, rather than a cause, of metal distribution in the organism. The bright orange luminescence of copper accumulated in copper cells of the midgut is severely reduced in the metallothionein gene family knockout, as well as in mutants of metal-responsive transcription factor 1 (MTF-1), the main regulator of metallothionein expression. This indicates that an in vivo metallothionein-copper complex forms the basis of this luminescence. Strikingly, metallothionein mutants show an increased, MTF-1-dependent induction of metallothionein promoters in response to copper, cadmium, silver, zinc, and mercury. We conclude that free metal, but not metallothionein-bound metal, triggers the activation of MTF-1 and that metallothioneins regulate their own expression by a negative feedback loop.
Figures



Similar articles
-
The four members of the Drosophila metallothionein family exhibit distinct yet overlapping roles in heavy metal homeostasis and detoxification.Genes Cells. 2006 Jun;11(6):647-58. doi: 10.1111/j.1365-2443.2006.00971.x. Genes Cells. 2006. PMID: 16716195
-
Dissection of Drosophila MTF-1 reveals a domain for differential target gene activation upon copper overload vs. copper starvation.Int J Biochem Cell Biol. 2012 Feb;44(2):404-11. doi: 10.1016/j.biocel.2011.11.016. Epub 2011 Nov 26. Int J Biochem Cell Biol. 2012. PMID: 22138226
-
Mercury and cadmium trigger expression of the copper importer Ctr1B, which enables Drosophila to thrive on heavy metal-loaded food.Biol Chem. 2009 Feb;390(2):109-13. doi: 10.1515/BC.2009.020. Biol Chem. 2009. PMID: 19040355
-
The taste of heavy metals: gene regulation by MTF-1.Biochim Biophys Acta. 2012 Sep;1823(9):1416-25. doi: 10.1016/j.bbamcr.2012.01.005. Epub 2012 Jan 20. Biochim Biophys Acta. 2012. PMID: 22289350 Review.
-
Regulation of metallothionein gene expression.Prog Food Nutr Sci. 1990;14(2-3):193-258. Prog Food Nutr Sci. 1990. PMID: 2293243 Review.
Cited by
-
Characterization of the role of the antioxidant proteins metallothioneins 1 and 2 in an animal model of Alzheimer's disease.Cell Mol Life Sci. 2012 Nov;69(21):3665-81. doi: 10.1007/s00018-012-1045-y. Epub 2012 Jul 6. Cell Mol Life Sci. 2012. PMID: 22766972 Free PMC article.
-
Meeting a threat of the Anthropocene: Taste avoidance of metal ions by Drosophila.Proc Natl Acad Sci U S A. 2022 Jun 21;119(25):e2204238119. doi: 10.1073/pnas.2204238119. Epub 2022 Jun 14. Proc Natl Acad Sci U S A. 2022. PMID: 35700364 Free PMC article.
-
Functional studies of Drosophila zinc transporters reveal the mechanism for dietary zinc absorption and regulation.BMC Biol. 2013 Sep 24;11:101. doi: 10.1186/1741-7007-11-101. BMC Biol. 2013. PMID: 24063361 Free PMC article.
-
Differential sexual survival of Drosophila melanogaster on copper sulfate.Genetica. 2017 Apr;145(2):131-137. doi: 10.1007/s10709-017-9951-4. Epub 2017 Feb 2. Genetica. 2017. PMID: 28154959
-
A role for the Drosophila zinc transporter Zip88E in protecting against dietary zinc toxicity.PLoS One. 2017 Jul 13;12(7):e0181237. doi: 10.1371/journal.pone.0181237. eCollection 2017. PLoS One. 2017. PMID: 28704512 Free PMC article.
References
-
- Abel, J., and N. de Ruiter. 1989. Inhibition of hydroxyl-radical-generated DNA degradation by metallothionein. Toxicol. Lett. 47:191-196. - PubMed
-
- Askwith, C., and J. Kaplan. 1998. Iron and copper transport in yeast and its relevance to human disease. Trends Biochem. Sci. 23:135-138. - PubMed
-
- Balamurugan, K., D. Egli, A. Selvaraj, B. Zhang, O. Georgiev, and W. Schaffner. 2004. Metal-responsive transcription factor (MTF-1) and heavy metal stress response in Drosophila and mammalian cells: a functional comparison. Biol. Chem. 385:597-603. - PubMed
-
- Ballan-Dufrancais, C. 2002. Localization of metals in cells of pterygote insects. Microsc. Res. Tech. 56:403-420. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
