FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG)
- PMID: 16508637
- PMCID: PMC2361370
- DOI: 10.1038/sj.bjc.6603011
FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG)
Abstract
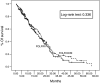
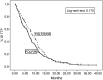
To compare the efficacy and toxicity of oxaliplatin (L-OHP) in combination with irinotecan (CPT-11), 5-fluorouracil (5-FU) and leucovorin (LV) (FOLFOXIRI) vs irinotecan and 5-FU/LV (FOLFIRI) as first-line treatment of patients with metastatic colorectal cancer (MCC). A total of 283 chemotherapy-naïve patients with MCC were enrolled (FOLFIRI arm: n=146; FOLFOXIRI arm: n=137). In the FOLFOXIRI arm, CPT-11 (150 mg m(-2)) was given on d1, L-OHP (65 mg m(-2)) on d2, LV (200 mg m(-2)) on days 2 and 3 and 5-FU (400 mg m(-2) as i.v. bolus and 600 mg m(-2) as 22 h i.v. continuous infusion) on days 2 and 3. In the FOLFIRI arm, CPT-11 (180 mg m(-2)) was given on d1 whereas LV and 5-FU were administered in the same way as in the FOLFOXIRI regimen. Both regimens were administered every 2 weeks. There was no difference in terms of overall survival (median OS: 19.5 and 21.5 months, for FOLFIRI and FOLFOXIRI, respectively; P=0.337), median time to disease progression (FOLFIRI: 6.9 and FOLFOXIRI: 8.4 months; P=0.17), response rates (33.6 and 43% for FOLFIRI and FOLFOXIRI, respectively; P=0.168). Patients treated with FOLFOXIRI had a significantly higher incidence of alopecia (P=0.0001), diarrhoea (P=0.0001) and neurosensory toxicity (P=0.001) compared with patients treated with FOLFIRI. The present study failed to demonstrate any superiority of the FOLFOXIRI combination compared with the FOLFIRI regimen, although the observed median OS is one of the best ever reported in the literature.
Figures
Similar articles
-
Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial.Lancet Oncol. 2020 Apr;21(4):497-507. doi: 10.1016/S1470-2045(19)30862-9. Epub 2020 Mar 9. Lancet Oncol. 2020. PMID: 32164906 Clinical Trial.
-
FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study.J Clin Oncol. 2004 Jan 15;22(2):229-37. doi: 10.1200/JCO.2004.05.113. Epub 2003 Dec 2. J Clin Oncol. 2004. PMID: 14657227 Clinical Trial.
-
Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest.J Clin Oncol. 2007 May 1;25(13):1670-6. doi: 10.1200/JCO.2006.09.0928. J Clin Oncol. 2007. PMID: 17470860 Clinical Trial.
-
Single-agent irinotecan or FOLFIRI as second-line chemotherapy for advanced colorectal cancer; results of a randomised phase II study (DaVINCI) and meta-analysis [corrected].Eur J Cancer. 2011 Aug;47(12):1826-36. doi: 10.1016/j.ejca.2011.04.024. Epub 2011 Jun 12. Eur J Cancer. 2011. PMID: 21665462 Clinical Trial.
-
The Clinical and Cost Effectiveness of Aflibercept in Combination with Irinotecan and Fluorouracil-Based Therapy (FOLFIRI) for the Treatment of Metastatic Colorectal Cancer Which has Progressed Following Prior Oxaliplatin-Based Chemotherapy: a Critique of the Evidence.Pharmacoeconomics. 2015 May;33(5):457-66. doi: 10.1007/s40273-015-0257-z. Pharmacoeconomics. 2015. PMID: 25616671 Review.
Cited by
-
What is the optimal neo-adjuvant treatment for liver metastasis?Ther Adv Med Oncol. 2013 Jul;5(4):221-34. doi: 10.1177/1758834013485111. Ther Adv Med Oncol. 2013. PMID: 23858331 Free PMC article.
-
Efficacy and quality of life for FOLFOX/bevacizumab +/- irinotecan in first-line metastatic colorectal cancer-final results of the AIO CHARTA trial.Br J Cancer. 2024 Feb;130(2):233-241. doi: 10.1038/s41416-023-02496-4. Epub 2023 Nov 23. Br J Cancer. 2024. PMID: 37996507 Free PMC article.
-
Role of oxidative stress in oxaliplatin-induced enteric neuropathy and colonic dysmotility in mice.Br J Pharmacol. 2016 Dec;173(24):3502-3521. doi: 10.1111/bph.13646. Epub 2016 Nov 16. Br J Pharmacol. 2016. PMID: 27714760 Free PMC article.
-
Concurrent chemoradiotherapy followed by metastasectomy converts to survival benefit in stage IV rectum cancer.J Gastrointest Surg. 2012 Oct;16(10):1888-96. doi: 10.1007/s11605-012-1959-6. Epub 2012 Jul 26. J Gastrointest Surg. 2012. PMID: 22833439
-
Prognostic significance of peritoneal metastasis from colorectal cancer treated with first-line triplet chemotherapy.World J Clin Cases. 2022 Mar 16;10(8):2429-2438. doi: 10.12998/wjcc.v10.i8.2429. World J Clin Cases. 2022. PMID: 35434075 Free PMC article. Clinical Trial.
References
-
- American Cancer Society. Cancer Facts and Figures. Atlanta, GA: American Cancer Society, 2005. http://www.cancer.org/downloads/STT/CAFF2005f4PWsecured.pdf. Assessed March 8, 2005
-
- Caussanel JP, Lévi F, Brienza S, Misset JL, Itzhaki M, Adam R, Milano G, Hecquet B, Mathe G (1990) Phase I trial of 5-day continuous venous infusion of oxaliplatin at circadian rhythm modulated rate compared with constant rate. J Natl Cancer Inst 82: 1046–1050 - PubMed
-
- Cox DR (1972) Regression models and life-tables. J R Stat Soc 34B: 187–220
-
- de Gramont A, Bosset JF, Milan C, Rougier P, Bouche O, Etienne PL, Morvan F, Louvet C, Guillot T, Francois E, Bedenne L (1997) Randomized trial comparing monthly low-dose leukovorin and fluorouracil bolus with bimonthly high dose leukovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 14: 808–815 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

