hTERT phosphorylation by PKC is essential for telomerase holoprotein integrity and enzyme activity in head neck cancer cells
- PMID: 16508638
- PMCID: PMC2361368
- DOI: 10.1038/sj.bjc.6603008
hTERT phosphorylation by PKC is essential for telomerase holoprotein integrity and enzyme activity in head neck cancer cells
Abstract
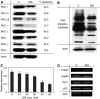
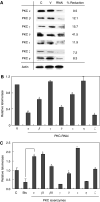
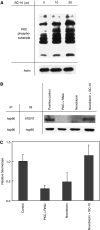
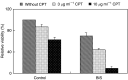
Telomerase activity is suppressed in normal somatic tissues but is activated in most cancer cells. We have previously found that all six telomerase subunit proteins, including hTERT and hsp90 are needed for full enzyme activity. Telomerase activity has been reported to be upregulated by protein kinase C (PKC), but the mechanism is not clear. In this study, we examined how PKC regulates telomerase activity in head and neck cancer cells. PKC inhibitor, bisindolylmaleimide I (BIS), inhibited telomerase activity but had no effect on the expressions of telomerase core subunits. RNA interference (RNAi) and in vitro phosphorylation studies revealed that PKC isoforms alpha, beta, delta, epsilon, zeta specifically involved in telomerase regulation, and the phosphorylation target was on hTERT. Treatment with the hsp-90 inhibitor novobiocin dissociated hsp90 and hTERT as revealed by immunoprecipitation and immunoblot analysis and reduced telomerase activity. Treatment with the PKC activator SC-10 restored the association of hsp90 and hTERT and reactivate telomerase, suggesting that hTERT phosphorylation by PKC is essential for telomerase holoenzyme integrity and function. Analysis on clinical normal and tumour tissues reveal that the expressions of PKC alpha, beta, delta, epsilon, zeta were higher in the tumour tissues, correlated with telomerase activity. Disruption of PKC phosphorylation by BIS significantly increased chemosensitivity to cisplatin. In conclusion, PKC isoenzymes alpha, beta, delta, epsilon, zeta regulate telomerase activity in head and neck cancer cells by phosphorylating hTERT. This phosphorylation is essential for telomerase holoenzyme assembly, leading to telomerase activation and oncogenesis. Manipulation of telomerase activity by PKC inhibitors is worth exploring as an adjuvant therapeutic approach.
Figures


Similar articles
-
Protein kinase C modulates telomerase activity in human cervical cancer cells.Exp Mol Med. 2001 Sep 30;33(3):156-63. doi: 10.1038/emm.2001.27. Exp Mol Med. 2001. PMID: 11642552
-
Telomerase is regulated by protein kinase C-zeta in human nasopharyngeal cancer cells.Biochem J. 2001 Apr 15;355(Pt 2):459-64. doi: 10.1042/0264-6021:3550459. Biochem J. 2001. PMID: 11284734 Free PMC article.
-
The dual role of protein kinase C in the regulation of telomerase activity in human lymphocytes.FEBS Lett. 2003 Apr 10;540(1-3):91-5. doi: 10.1016/s0014-5793(03)00230-8. FEBS Lett. 2003. PMID: 12681489
-
A major role of PKC theta and NFkappaB in the regulation of hTERT in human T lymphocytes.FEBS Lett. 2006 Dec 22;580(30):6819-24. doi: 10.1016/j.febslet.2006.11.044. Epub 2006 Nov 27. FEBS Lett. 2006. PMID: 17141225
-
[Molecular mechanisms involved in the regulation of telomerase activity].Sheng Li Ke Xue Jin Zhan. 2001 Jul;32(3):220-4. Sheng Li Ke Xue Jin Zhan. 2001. PMID: 12545793 Review. Chinese.
Cited by
-
Telomerase inhibition decreases alpha-fetoprotein expression and secretion by hepatocellular carcinoma cell lines: in vitro and in vivo study.PLoS One. 2015 Mar 30;10(3):e0119512. doi: 10.1371/journal.pone.0119512. eCollection 2015. PLoS One. 2015. PMID: 25822740 Free PMC article.
-
Inhibition of protein kinase C enhances angiogenesis induced by platelet-derived growth factor C in hyperglycemic endothelial cells.Cardiovasc Diabetol. 2015 Feb 7;14:19. doi: 10.1186/s12933-015-0180-9. Cardiovasc Diabetol. 2015. PMID: 25849290 Free PMC article.
-
Diverse regulatory manners of human telomerase reverse transcriptase.Cell Commun Signal. 2019 Jun 11;17(1):63. doi: 10.1186/s12964-019-0372-0. Cell Commun Signal. 2019. PMID: 31186051 Free PMC article. Review.
-
Human telomerase activity regulation.Mol Biol Rep. 2011 Jun;38(5):3339-49. doi: 10.1007/s11033-010-0439-x. Epub 2010 Nov 18. Mol Biol Rep. 2011. PMID: 21086176 Free PMC article. Review.
-
The intrabody targeting of hTERT attenuates the immortality of cancer cells.Cell Mol Biol Lett. 2010;15(1):32-45. doi: 10.2478/s11658-009-0032-2. Epub 2009 Sep 23. Cell Mol Biol Lett. 2010. PMID: 19774346 Free PMC article.
References
-
- Abbas T, White D, Hui L, Yoshida K, Foster DA, Bargonetti J (2004) Inhibition of human p53 basal transcription by down-regulation of protein kinase Cdelta. J Biol Chem 279: 9970–9979 - PubMed
-
- Breitschopf K, Zeiher AM, Dimmeler S (2001) Pro-atherogenic factors induce telomerase inactivation in endothelial cells through an Akt-dependent mechanism. FEBS Lett 493: 21–25 - PubMed
-
- Chang JT, Chen YL, Yang HT, Chen CY, Cheng AJ (2002) Differential regulation of telomerase activity by six telomerase subunits. Eur J Biochem 269: 3442–3450 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

