A phase III trial comparing CHOP to PMitCEBO with or without G-CSF in patients aged 60 plus with aggressive non-Hodgkin's lymphoma
- PMID: 16508640
- PMCID: PMC3216418
- DOI: 10.1038/sj.bjc.6602975
A phase III trial comparing CHOP to PMitCEBO with or without G-CSF in patients aged 60 plus with aggressive non-Hodgkin's lymphoma
Abstract
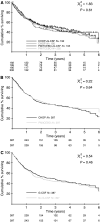
The management of older patients with aggressive non-Hodgkin's lymphoma presents a challenge to the physician. Age is a poor prognostic indicator, due to reduced ability to tolerate and maintain dose-intensive chemotherapy. Generally, older patients demonstrate a lower response rate, reduced survival and increased toxicity, although the majority of large randomised trials exclude older patients. This randomised trial was conducted in patients 60 years or over to compare CHOP (cyclophosphamide 750 mg m(-2), doxorubicin 50 mg m(-2), vincristine 1.4 mg m(-2), prednisolone 100 mg) with PMitCEBO (mitoxantrone 7 mg m(-2), cyclophosphamide 300 mg m(-2), etoposide 150 mg m(-2), vincristine 1.4 mg m(-2), bleomycin 10 mg m(-2) and prednisolone 50 mg). Due to the myelosuppressive nature of these regimens, patients were also randomised to the addition of G-CSF. The formal results of this trial with long-term follow-up are now reported. Data were analysed to assess efficacy and toxicity. Overall response rate was 84% in the CHOP arm and 83% in the PMitCEBO arm, with overall response rates of 83% for the use of G-CSF and 84% for no G-CSF. At median 44 months follow-up, there was no significant difference in failure-free, progression-free or overall survival between the CHOP and PMitCEBO arms. At 3 years, the actuarial failure-free survival was 44% in CHOP recipients and 42% in PMitCEBO recipients and the 3-year actuarial overall survival was 46% and 45% respectively. There was no significant difference in the failure-free, progression-free or overall survival with the addition of G-CSF.
Figures

References
-
- Armitage J, Potter J (1984) Aggressive chemotherapy for diffuse histiocytic lymphoma in the elderly: increased complications with advancing age. J Am Geriatr Soc 32: 269–273 - PubMed
-
- Balducci L, Lyman G, Ozer H (2001) Patients aged {⩾} 70 are at high risk for neutropenic infection and should receive hemopoietic growth. J Clin Oncol 19: 1583–1585 - PubMed
-
- Bastion Y, Blay JY, Divine M, Brice P, Bordessoule D, Sebban C, Blanc M, Tilly H, Lederlin P, Deconinck E, Salles B, Dumontet C, Briere J, Coiffier B (1997) Elderly patients with aggressive non-Hodgkin's lymphoma: disease presentation, response to treatment, and survival – a Groupe d'Etude des Lymphomes de l'Adulte study on 453 patients older than 69 years. J Clin Oncol 15: 2945–2953 - PubMed
-
- Bertini M, Freilone R, Vitolo U Botto B, Pizzuti M, Gavarotti P, Levis A, Orlandi E, Orsucci L, Pini M (1994) P-VEBEC: a new 8-weekly schedule with or without rG-CSF for elderly patients with aggressive non-Hodgkin's lymphoma. Ann Oncol 5: 895–900 - PubMed
-
- 2001 Census (2001) Office of Population Censuses and Surveys. London, UK: Her Majesty's Stationery Office
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

