Gender and post-ischemic recovery of hypertrophied rat hearts
- PMID: 16509993
- PMCID: PMC1413556
- DOI: 10.1186/1471-2261-6-8
Gender and post-ischemic recovery of hypertrophied rat hearts
Abstract
Background: Gender influences the cardiac response to prolonged increases in workload, with differences at structural, functional, and molecular levels. However, it is unknown if post-ischemic function or metabolism of female hypertrophied hearts differ from male hypertrophied hearts. Thus, we tested the hypothesis that gender influences post-ischemic function of pressure-overload hypertrophied hearts and determined if the effect of gender on post-ischemic outcome could be explained by differences in metabolism, especially the catabolic fate of glucose.
Methods: Function and metabolism of isolated working hearts from sham-operated and aortic-constricted male and female Sprague-Dawley rats before and after 20 min of no-flow ischemia (N = 17 to 27 per group) were compared. Parallel series of hearts were perfused with Krebs-Henseleit solution containing 5.5 mM [5-3H/U-14C]-glucose, 1.2 mM [1-14C]-palmitate, 0.5 mM [U-14C]-lactate, and 100 mU/L insulin to measure glycolysis and glucose oxidation in one series and oxidation of palmitate and lactate in the second. Statistical analysis was performed using two-way analysis of variance. The sequential rejective Bonferroni procedure was used to correct for multiple comparisons and tests.
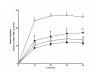
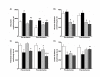
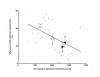
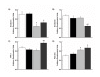
Results: Female gender negatively influenced post-ischemic function of non-hypertrophied hearts, but did not significantly influence function of hypertrophied hearts after ischemia such that mass-corrected hypertrophied heart function did not differ between genders. Before ischemia, glycolysis was accelerated in hypertrophied hearts, but to a greater extent in males, and did not differ between male and female non-hypertrophied hearts. Glycolysis fell in all groups after ischemia, except in non-hypertrophied female hearts, with the reduction in glycolysis after ischemia being greatest in males. Post-ischemic glycolytic rates were, therefore, similarly accelerated in hypertrophied male and female hearts and higher in female than male non-hypertrophied hearts. Glucose oxidation was lower in female than male hearts and was unaffected by hypertrophy or ischemia. Consequently, non-oxidative catabolism of glucose after ischemia was lowest in male non-hypertrophied hearts and comparably elevated in hypertrophied hearts of both sexes. These differences in non-oxidative glucose catabolism were inversely related to post-ischemic functional recovery.
Conclusion: Gender does not significantly influence post-ischemic function of hypertrophied hearts, even though female sex is detrimental to post-ischemic function in non-hypertrophied hearts. Differences in glucose catabolism may contribute to hypertrophy-induced and gender-related differences in post-ischemic function.
Figures


Similar articles
-
Regression of cardiac hypertrophy normalizes glucose metabolism and left ventricular function during reperfusion.J Mol Cell Cardiol. 1997 Mar;29(3):939-48. doi: 10.1006/jmcc.1996.0336. J Mol Cell Cardiol. 1997. PMID: 9152855
-
Accelerated glycolysis and greater postischemic dysfunction in hypertrophied rat hearts are independent of coronary flow.Can J Cardiol. 2001 Aug;17(8):889-94. Can J Cardiol. 2001. PMID: 11521131
-
Glucose utilization and glycogen turnover are accelerated in hypertrophied rat hearts during severe low-flow ischemia.J Mol Cell Cardiol. 1999 Mar;31(3):493-502. doi: 10.1006/jmcc.1998.0804. J Mol Cell Cardiol. 1999. PMID: 10198181
-
Energy metabolism in the hypertrophied heart.Heart Fail Rev. 2002 Apr;7(2):161-73. doi: 10.1023/a:1015380609464. Heart Fail Rev. 2002. PMID: 11988640 Review.
-
Contribution of protons to post-ischemic Na(+) and Ca(2+) overload and left ventricular mechanical dysfunction.J Cardiovasc Electrophysiol. 2006 May;17 Suppl 1:S141-S148. doi: 10.1111/j.1540-8167.2006.00395.x. J Cardiovasc Electrophysiol. 2006. PMID: 16686669 Review.
Cited by
-
Dichloroacetate selectively improves cardiac function and metabolism in female and male rainbow trout.Am J Physiol Heart Circ Physiol. 2014 Nov 15;307(10):H1401-11. doi: 10.1152/ajpheart.00755.2013. Epub 2014 Sep 12. Am J Physiol Heart Circ Physiol. 2014. PMID: 25217653 Free PMC article.
-
Mice over-expressing the myocardial creatine transporter develop progressive heart failure and show decreased glycolytic capacity.J Mol Cell Cardiol. 2010 Apr;48(4):582-90. doi: 10.1016/j.yjmcc.2009.10.033. Epub 2009 Nov 11. J Mol Cell Cardiol. 2010. PMID: 19913546 Free PMC article.
-
Cardioprotection in females: a role for nitric oxide and altered gene expression.Heart Fail Rev. 2007 Dec;12(3-4):293-300. doi: 10.1007/s10741-007-9035-0. Heart Fail Rev. 2007. PMID: 17508281 Review.
-
Age and ovariectomy abolish beneficial effects of female sex on rat ventricular myocytes exposed to simulated ischemia and reperfusion.PLoS One. 2012;7(6):e38425. doi: 10.1371/journal.pone.0038425. Epub 2012 Jun 6. PLoS One. 2012. PMID: 22701638 Free PMC article.
-
Functional polymorphism of the renalase gene is associated with cardiac hypertrophy in female patients with aortic stenosis.PLoS One. 2017 Oct 24;12(10):e0186729. doi: 10.1371/journal.pone.0186729. eCollection 2017. PLoS One. 2017. PMID: 29065134 Free PMC article.
References
-
- Carroll JD, Carroll EP, Feldman T, Ward DM, Lang RM, McGaughey D, Karp RB. Sex-associated differences in left ventricular function in aortic stenosis of the elderly. Circulation. 1992;86:1099–1107. - PubMed
-
- Villari B, Campbell SE, Schneider J, Vassalli G, Chiariello M, Hess OM. Sex-dependent differences in left ventricular function and structure in chronic pressure overload. Eur Heart J. 1995;16:1410–1419. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

