Coordinated requirements of human topo II and cohesin for metaphase centromere alignment under Mad2-dependent spindle checkpoint surveillance
- PMID: 16510521
- PMCID: PMC1446084
- DOI: 10.1091/mbc.e05-11-1089
Coordinated requirements of human topo II and cohesin for metaphase centromere alignment under Mad2-dependent spindle checkpoint surveillance
Abstract
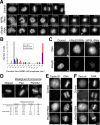
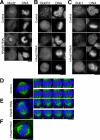
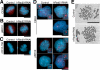
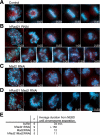
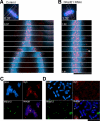
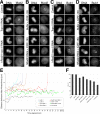
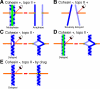
Cohesin maintains sister chromatid cohesion until its Rad21/Scc1/Mcd1 is cleaved by separase during anaphase. DNA topoisomerase II (topo II) maintains the proper topology of chromatid DNAs and is essential for chromosome segregation. Here we report direct observations of mitotic progression in individual HeLa cells after functional disruptions of hRad21, NIPBL, a loading factor for hRad21, and topo II alpha,beta by RNAi and a topo II inhibitor, ICRF-193. Mitosis is delayed in a Mad2-dependent manner after disruption of either or both cohesin and topo II. In hRad21 depletion, interphase pericentric architecture becomes aberrant, and anaphase is virtually permanently delayed as preseparated chromosomes are misaligned on the metaphase spindle. Topo II disruption perturbs centromere organization leading to intense Bub1, but no Mad2, on kinetochores and sustains a Mad2-dependent delay in anaphase onset with persisting securin. Thus topo II impinges upon centromere/kinetochore function. Disruption of topo II by RNAi or ICRF-193 overrides the mitotic delay induced by cohesin depletion: sister centromeres are aligned and anaphase spindle movements occur. The ensuing accumulation of catenations in preseparated sister chromatids may overcome the reduced tension arising from cohesin depletion, causing the override. Cohesin and topo II have distinct, yet coordinated functions in metaphase alignment.
Figures

References
-
- Atienza, J. M., Roth, R. B., Rosette, C., Smylie, K. J., Kammerer, S., Rehbock, J., Ekblom, J., and Denissenko, M. F. (2005). Suppression of RAD21 gene expression decreases cell growth and enhances cytotoxicity of etoposide and bleomycin in human breast cancer cells. Mol. Cancer Ther. 4, 361–368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

