Motion matters: secretory granule motion adjacent to the plasma membrane and exocytosis
- PMID: 16510523
- PMCID: PMC1446096
- DOI: 10.1091/mbc.e05-10-0938
Motion matters: secretory granule motion adjacent to the plasma membrane and exocytosis
Abstract
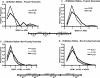
Total internal reflection fluorescence microscopy was used to monitor changes in individual granule motions related to the secretory response in chromaffin cells. Because the motions of granules are very small (tens of nanometers), instrumental noise in the quantitation of granule motion was taken into account. ATP and Ca2+, both of which prime secretion before fusion, also affect granule motion. Removal of ATP in permeabilized cells causes average granule motion to decrease. Nicotinic stimulation causes a calcium-dependent increase in average granule motion. This effect is more pronounced for granules that undergo exocytosis than for those that do not. Fusion is not preceded by a reduction in mobility. Granules sometimes move 100 nm or more up to and within a tenth of a second before fusion. Thus, the jittering motion of granules adjacent to the plasma membrane is regulated by factors that regulate secretion and may play a role in secretion. Motion continues until shortly before fusion, suggesting that interaction of granule and plasma membrane proteins is transient. Disruption of actin dynamics did not significantly alter granule motion.
Figures


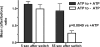
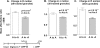



References
-
- Axelrod, D. (2001). Selective imaging of surface fluorescence with very high aperture microscope objectives. J. Biomed. Opt. 6, 6-13. - PubMed
-
- Axelrod, D. (2003). Total internal reflection fluorescence microscopy in cell biology. Methods Enzymol. 361, 1-33. - PubMed
-
- Axelrod, D., Burghardt, T. P., and Thompson, N. L. (1984). Total internal reflection fluorescence. Annu. Rev. Biophys. Bioeng. 13, 247-268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

