Quantitative trait loci controlling refractoriness to Plasmodium falciparum in natural Anopheles gambiae mosquitoes from a malaria-endemic region in western Kenya
- PMID: 16510784
- PMCID: PMC1461423
- DOI: 10.1534/genetics.105.055129
Quantitative trait loci controlling refractoriness to Plasmodium falciparum in natural Anopheles gambiae mosquitoes from a malaria-endemic region in western Kenya
Abstract
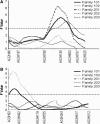
Natural anopheline populations exhibit much variation in ability to support malaria parasite development, but the genetic mechanisms underlying this variation are not clear. Previous studies in Mali, West Africa, identified two quantitative trait loci (QTL) in Anopheles gambiae mosquitoes that confer refractoriness (failure of oocyst development in mosquito midguts) to natural Plasmodium falciparum parasites. We hypothesize that new QTL may be involved in mosquito refractoriness to malaria parasites and that the frequency of natural refractoriness genotypes may be higher in the basin region of Lake Victoria, East Africa, where malaria transmission intensity and parasite genetic diversity are among the highest in the world. Using field-derived F2 isofemale families and microsatellite marker genotyping, two loci significantly affecting oocyst density were identified: one on chromosome 2 between markers AG2H135 and AG2H603 and the second on chromosome 3 near marker AG3H93. The first locus was detected in three of the five isofemale families studied and colocalized to the same region as Pen3 and pfin1 described in other studies. The second locus was detected in two of the five isofemale families, and it appears to be a new QTL. QTL on chromosome 2 showed significant additive effects while those on chromosome 3 exhibited significant dominant effects. Identification of P. falciparum-refractoriness QTL in natural An. gambiae mosquitoes is critical to the identification of the genes involved in malaria parasite transmission in nature and for understanding the coevolution between malaria parasites and mosquito vectors.
Figures
References
-
- Aultmann, K. S., B. J. Beaty and E. D. Walker, 2001. Genetically manipulated vectors of human disease: a practical overview. Trends Parasitol. 17: 507–509. - PubMed
-
- Barillas-Mury, C., B. Wizel and Y. S. Han, 2000. Mosquito immune responses and implications in malaria transmission: lessons from insect model systems and implications in vertebrate innate immunity and vaccine development. Insect Biochem. Mol. Biol. 30: 429–442. - PubMed
-
- Beier, J. C., G. F. Killeen and J. I. Githure, 1999. Entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am. J. Trop. Med. Hyg. 61: 109–113. - PubMed
-
- Blandin, S., S. H. Shiao, L. F. Moita, C. J. Janse, A. P. Waters et al., 2004. Complement-like protein TEP1 is a determinant of vectorial capacity in malaria vector Anopheles gambiae. Cell 116: 661–670. - PubMed
-
- Breman, J. G., M. S. Alilio and A. Mills, 2004. Conquering the intolerable burden of malaria: what's new, what's needed: a summary. Am. J. Trop. Med. Hyg. 71: 1–15. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

