The hepatitis C virus 3'-untranslated region or a poly(A) tract promote efficient translation subsequent to the initiation phase
- PMID: 16510853
- PMCID: PMC1388098
- DOI: 10.1093/nar/gkl019
The hepatitis C virus 3'-untranslated region or a poly(A) tract promote efficient translation subsequent to the initiation phase
Abstract
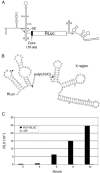
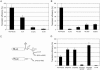
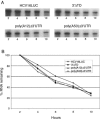
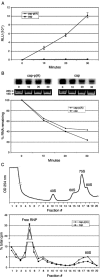
Enhancement of eukaryotic messenger RNA (mRNA) translation initiation by the 3' poly(A) tail is mediated through interaction of poly(A)-binding protein with eukaryotic initiation factor (eIF) 4G, bridging the 5' terminal cap structure. In contrast to cellular mRNA, translation of the uncapped, non-polyadenylated hepatitis C virus (HCV) genome occurs independently of eIF4G and a role for 3'-untranslated sequences in modifying HCV gene expression is controversial. Utilizing cell-based and in vitro translation assays, we show that the HCV 3'-untranslated region (UTR) or a 3' poly(A) tract of sufficient length interchangeably stimulate translation dependent upon the HCV internal ribosomal entry site (IRES). However, in contrast to cap-dependent translation, the rate of initiation at the HCV IRES was unaffected by 3'-untranslated sequences. Analysis of post-initiation events revealed that the 3' poly(A) tract and HCV 3'-UTR improve translation efficiency by enabling termination and possibly ribosome recycling for successive rounds of translation.
Figures



References
-
- Hershey J.W.B., Merrick W.C. The pathway and mechanism of initiation of protein synthesis. In: Sonenberg N., Hershey J.W.B., Mathews M.B., editors. Translational Control of Gene Expression. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 33–88.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

