Role of electrode design on the volume of tissue activated during deep brain stimulation
- PMID: 16510937
- PMCID: PMC2583360
- DOI: 10.1088/1741-2560/3/1/001
Role of electrode design on the volume of tissue activated during deep brain stimulation
Abstract
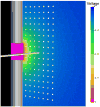
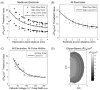
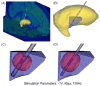
Deep brain stimulation (DBS) is an established clinical treatment for a range of neurological disorders. Depending on the disease state of the patient, different anatomical structures such as the ventral intermediate nucleus of the thalamus (VIM), the subthalamic nucleus or the globus pallidus are targeted for stimulation. However, the same electrode design is currently used in nearly all DBS applications, even though substantial morphological and anatomical differences exist between the various target nuclei. The fundamental goal of this study was to develop a theoretical understanding of the impact of changes in the DBS electrode contact geometry on the volume of tissue activated (VTA) during stimulation. Finite element models of the electrodes and surrounding medium were coupled to cable models of myelinated axons to predict the VTA as a function of stimulation parameter settings and electrode design. Clinical DBS electrodes have cylindrical contacts 1.27 mm in diameter (d) and 1.5 mm in height (h). Our results show that changes in contact height and diameter can substantially modulate the size and shape of the VTA, even when contact surface area is preserved. Electrode designs with a low aspect ratio (d/h) maximize the VTA by providing greater spread of the stimulation parallel to the electrode shaft without sacrificing lateral spread. The results of this study provide the foundation necessary to customize electrode design and VTA shape for specific anatomical targets, and an example is presented for the VIM. A range of opportunities exist to engineer DBS systems to maximize stimulation of the target area while minimizing stimulation of non-target areas. Therefore, it may be possible to improve therapeutic benefit and minimize side effects from DBS with the design of target-specific electrodes.
Figures

References
-
- Benabid AL, Pollak P, Gao D, Hoffmann D, Limousin P, Gay E, Payen I, Benazzouz A. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996;84:203–14. - PubMed
-
- Butson CR, Hall J, Henderson JM, McIntyre CC. Patient-specific models of deep brain stimulation: 3D visualization of anatomy, electrode and volume of activation as a function of the stimulation parameters. Soc Neurosci Abstr 1011.11 2004
-
- Butson CR, Maks CB, Cooper SE, Henderson JM, McIntyre CC. Deep brain stimulation interactive visualization system. Soc Neurosci Abstr 898.7 2005b
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
