Mimivirus TyrRS: preliminary structural and functional characterization of the first amino-acyl tRNA synthetase found in a virus
- PMID: 16510997
- PMCID: PMC1952258
- DOI: 10.1107/S174430910500062X
Mimivirus TyrRS: preliminary structural and functional characterization of the first amino-acyl tRNA synthetase found in a virus
Abstract
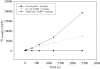
The amoeba-infecting Mimivirus is the largest known double-stranded DNA virus, with a 400 nm particle size, comparable to that of mycoplasma. The complete sequence of its 1.2 Mbp genome has recently been determined [Raoult et al. (2004), Science, 306, 1344-1350] and revealed numerous genes that were not expected to be found in a virus, such as genes encoding translation components, including 4-amino-acyl tRNA synthetases and homologues to various translation initiation, elongation and termination factors. A comprehensive structural and functional study of these Mimivirus gene products was initiated, as they may hold important clues about the origin of DNA viruses. Here, the first preliminary crystallographic and functional results obtained on one of these targets, Mimivirus TyrRS, are reported. Preliminary phasing was obtained using an original combination of homology modelling and normal mode analysis. Experimental evidence that Mimivirus tyrosyl tRNA synthetase recombinant gene product does indeed activate tyrosine is also presented.
Figures

References
-
- Abergel, C., Coutard, B., Byrne, D., Chenivesse, S., Claude, J.-B., Deregnaucourt, C., Fricaux, T., Gianesini-Boutreux, C., Jeudy, S., Lebrun, R., Maza, C., Notredame, C., Poirot, O., Suhre, K., Varagnol, M. & Claverie, J.-M. (2003). J. Struct. Funct. Genomics, 4, 141–157. - PubMed
-
- Audic, S., Lopez, F., Claverie, J.-M., Poirot, O. & Abergel, C. (1997). Proteins, 29, 251–256. - PubMed
-
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

