Precipitation diagram and optimization of crystallization conditions at low ionic strength for deglycosylated dye-decolorizing peroxidase from a basidiomycete
- PMID: 16511141
- PMCID: PMC1952364
- DOI: 10.1107/S1744309105019469
Precipitation diagram and optimization of crystallization conditions at low ionic strength for deglycosylated dye-decolorizing peroxidase from a basidiomycete
Abstract
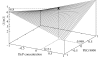
The growth of suitably sized protein crystals is essential for protein structure determination by X-ray crystallography. In general, crystals are grown using a trial-and-error method. However, these methods have been modified with the advent of microlitre dispensing-robot technology and of protocols that rapidly screen for crystal nucleation conditions. The use of one such automatic dispenser for mixing protein drops (1.3-2.0 microl in volume) of known concentration and pH with precipitating solutions (ejecting 2.0 microl droplets) containing salt is described here. The results of the experiments are relevant to a crystallization approach based on a two-step procedure: screening for the crystal nucleation step employing robotics followed by optimization of the crystallization conditions using incomplete factorial experimental design. Large crystals have successfully been obtained using quantities as small as 3.52 mg protein.
Figures


References
-
- Baldock, P., Mills, V. & Shaw Stewart, P. D. (1996). J. Cryst. Growth, 168, 170–174.
-
- Carter, C. W. Jr & Carter, C. W. (1979). J. Biol. Chem.254, 12219–12223. - PubMed
-
- Carter, C. W. Jr & Yin, Y. (1994). Acta Cryst. D50, 572–590. - PubMed
-
- Chayen, N. E. (1996). Protein Eng.9, 927–929. - PubMed
-
- Chayen, N. E. (1997). Structure, 5, 1269–1274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

