Crystallization of a functionally intact Hsc70 chaperone
- PMID: 16511258
- PMCID: PMC2150933
- DOI: 10.1107/S1744309105040303
Crystallization of a functionally intact Hsc70 chaperone
Abstract
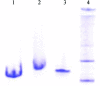
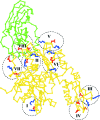
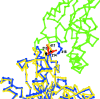
Hsp70s are essential chaperones with roles in a variety of cellular processes and representatives in all kingdoms of life. They are comprised of a nucleotide-binding domain (NBD) and a protein substrate-binding domain (SBD). Structures of isolated NBDs and SBDs have been reported but, until recently, a functionally intact Hsp70 containing both the NBD and SBD has resisted structure determination. Here, it is reported that preparation of diffraction-quality crystals of functionally intact bovine Hsc70 required (i) deletion of part of the protein to reduce oligomerization, (ii) point mutations in the interface between the SBD and NBD and (iii) use of high concentrations of the structure-stabilizing agents glycerol and trimethylamine oxide (TMAO). The introduction of point mutations in interdomain interfaces and the use of the potent structure stabilizer TMAO may be generally useful in crystallization of multidomain proteins that exhibit interdomain motions.
Figures



References
-
- Banecki, B., Zylicz, M., Bertoli, E. & Tanfani, F. (1992). J. Biol. Chem.267, 25051–25058. - PubMed
-
- Baskakov, I. V. & Bolen, D. W. (1998). J. Biol. Chem.273, 4831–4834. - PubMed
-
- Baskakov, I. V., Kumar, R., Srinivasan, G., Ji, Y.-S., Bolen, D. W. & Thompson, E. B. (1999). J. Biol. Chem.274, 10693–10696. - PubMed
-
- Boice, J. A. & Hightower, L. E. (1997). J. Biol Chem.272, 24825–24831. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

