A preliminary neutron diffraction study of rasburicase, a recombinant urate oxidase enzyme, complexed with 8-azaxanthin
- PMID: 16511330
- PMCID: PMC2197182
- DOI: 10.1107/S1744309106006439
A preliminary neutron diffraction study of rasburicase, a recombinant urate oxidase enzyme, complexed with 8-azaxanthin
Abstract
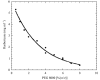
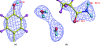
Crystallization and preliminary neutron diffraction measurements of rasburicase, a recombinant urate oxidase enzyme expressed by a genetically modified Saccharomyces cerevisiae strain, complexed with a purine-type inhibitor (8-azaxanthin) are reported. Neutron Laue diffraction data were collected to 2.1 A resolution using the LADI instrument from a crystal (grown in D2O) with volume 1.8 mm3. The aim of this neutron diffraction study is to determine the protonation states of the inhibitor and residues within the active site. This will lead to improved comprehension of the enzymatic mechanism of this important enzyme, which is used as a protein drug to reduce toxic uric acid accumulation during chemotherapy. This paper illustrates the high quality of the neutron diffraction data collected, which are suitable for high-resolution structural analysis. In comparison with other neutron protein crystallography studies to date in which a hydrogenated protein has been used, the volume of the crystal was relatively small and yet the data still extend to high resolution. Furthermore, urate oxidase has one of the largest primitive unit-cell volumes (space group I222, unit-cell parameters a = 80, b = 96, c = 106 A) and molecular weights (135 kDa for the homotetramer) so far successfully studied with neutrons.
Figures

Similar articles
-
Neutron Laue macromolecular crystallography.Eur Biophys J. 2006 Sep;35(7):611-20. doi: 10.1007/s00249-006-0074-6. Epub 2006 Aug 3. Eur Biophys J. 2006. PMID: 16897039 Review.
-
The neutron structure of urate oxidase resolves a long-standing mechanistic conundrum and reveals unexpected changes in protonation.PLoS One. 2014 Jan 23;9(1):e86651. doi: 10.1371/journal.pone.0086651. eCollection 2014. PLoS One. 2014. PMID: 24466188 Free PMC article.
-
Urate oxidase from Aspergillus flavus: new crystal-packing contacts in relation to the content of the active site.Acta Crystallogr D Biol Crystallogr. 2005 Mar;61(Pt 3):218-29. doi: 10.1107/S0907444904031531. Epub 2005 Feb 24. Acta Crystallogr D Biol Crystallogr. 2005. PMID: 15735331
-
Large crystal growth by thermal control allows combined X-ray and neutron crystallographic studies to elucidate the protonation states in Aspergillus flavus urate oxidase.J R Soc Interface. 2009 Oct 6;6 Suppl 5(Suppl 5):S599-610. doi: 10.1098/rsif.2009.0162.focus. Epub 2009 Jul 8. J R Soc Interface. 2009. PMID: 19586953 Free PMC article. Review.
-
High pressure macromolecular crystallography: the 140-MPa crystal structure at 2.3 A resolution of urate oxidase, a 135-kDa tetrameric assembly.Biochim Biophys Acta. 2006 Mar;1764(3):391-7. doi: 10.1016/j.bbapap.2006.01.006. Epub 2006 Jan 26. Biochim Biophys Acta. 2006. PMID: 16478683
Cited by
-
A preliminary neutron crystallographic study of thaumatin.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008 May 1;64(Pt 5):378-81. doi: 10.1107/S1744309108008294. Epub 2008 Apr 5. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008. PMID: 18453706 Free PMC article.
-
Neutron Laue macromolecular crystallography.Eur Biophys J. 2006 Sep;35(7):611-20. doi: 10.1007/s00249-006-0074-6. Epub 2006 Aug 3. Eur Biophys J. 2006. PMID: 16897039 Review.
-
Neutron crystallography: opportunities, challenges, and limitations.Curr Opin Struct Biol. 2008 Oct;18(5):593-600. doi: 10.1016/j.sbi.2008.06.009. Epub 2008 Aug 7. Curr Opin Struct Biol. 2008. PMID: 18656544 Free PMC article. Review.
-
Optimization of crystallization of biological macromolecules using dialysis combined with temperature control.J Appl Crystallogr. 2020 May 5;53(Pt 3):686-698. doi: 10.1107/S1600576720003209. eCollection 2020 Jun 1. J Appl Crystallogr. 2020. PMID: 32684884 Free PMC article.
-
The neutron structure of urate oxidase resolves a long-standing mechanistic conundrum and reveals unexpected changes in protonation.PLoS One. 2014 Jan 23;9(1):e86651. doi: 10.1371/journal.pone.0086651. eCollection 2014. PLoS One. 2014. PMID: 24466188 Free PMC article.
References
-
- Arzt, S., Campbell, J. W., Harding, M. M., Hao, Q. & Helliwell, J. R. (1999). J. Appl. Cryst.32, 554–562.
-
- Bau, R. (2004). J. Synchrotron Rad.11, 76–79. - PubMed
-
- Bennett, B. C., Meilleur, F., Myles, D. A., Howell, E. E. & Dealwis, C. G. (2005). Acta Cryst. D61, 574–579. - PubMed
-
- Bonneté, F., Vivarès, D., Robert, C. & Colloc’h, N. (2001). J. Cryst. Growth, 232, 330–339.
-
- Budayova-Spano, M., Lafont, S., Astier, J. P., Ebel, C. & Veesler, S. (2000). J. Cryst. Growth, 217, 311–319.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

