Oncogenic function for the Dlg1 mammalian homolog of the Drosophila discs-large tumor suppressor
- PMID: 16511562
- PMCID: PMC1422156
- DOI: 10.1038/sj.emboj.7601030
Oncogenic function for the Dlg1 mammalian homolog of the Drosophila discs-large tumor suppressor
Abstract
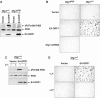
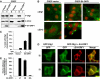
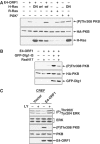
The fact that several different human virus oncoproteins, including adenovirus type 9 E4-ORF1, evolved to target the Dlg1 mammalian homolog of the membrane-associated Drosophila discs-large tumor suppressor has implicated this cellular factor in human cancer. Despite a general belief that such interactions function solely to inactivate this suspected human tumor suppressor protein, we demonstrate here that E4-ORF1 specifically requires endogenous Dlg1 to provoke oncogenic activation of phosphatidylinositol 3-kinase (PI3K) in cells. Based on our results, we propose a model wherein E4-ORF1 binding to Dlg1 triggers the resulting complex to translocate to the plasma membrane and, at this site, to promote Ras-mediated PI3K activation. These findings establish the first known function for Dlg1 in virus-mediated cellular transformation and also surprisingly expose a previously unrecognized oncogenic activity encoded by this suspected cellular tumor suppressor gene.
Figures




References
-
- Auger KR, Wang J, Narsimhan RP, Holcombe T, Roberts TM (2000) Constitutive cellular expression of PI 3-kinase is distinct from transient expression. Biochem Biophys Res Commun 272: 822–829 - PubMed
-
- Barmak K, Harhaj E, Grant C, Alefantis T, Wigdahl B (2003) Human T cell leukemia virus type I-induced disease: pathways to cancer and neurodegeneration. Virology 308: 1–12 - PubMed
-
- Bell JA, Rinchik EM, Raymond S, Suffolk R, Jackson IJ (1995) A high-resolution map of the brown (b, Tyrp1) deletion complex of mouse chromosome 4. Mamm Genome 6: 389–395 - PubMed
-
- Cavatorta AL, Fumero G, Chouhy D, Aguirre R, Nocito AL, Giri AA, Banks L, Gardiol D (2004) Differential expression of the human homologue of Drosophila discs large oncosuppressor in histologic samples from human papillomavirus-associated lesions as a marker for progression to malignancy. Int J Cancer 111: 373–380 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

