The expanding transcriptome: the genome as the 'Book of Sand'
- PMID: 16511566
- PMCID: PMC1409726
- DOI: 10.1038/sj.emboj.7601023
The expanding transcriptome: the genome as the 'Book of Sand'
Abstract
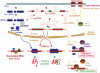
The central dogma of molecular biology inspired by classical work in prokaryotic organisms accounts for only part of the genetic agenda of complex eukaryotes. First, post-transcriptional events lead to the generation of multiple mRNAs, proteins and functions from a single primary transcript, revealing regulatory networks distinct in mechanism and biological function from those controlling RNA transcription. Second, a variety of populous families of small RNAs (small nuclear RNAs, small nucleolar RNAs, microRNAs, siRNAs and shRNAs) assemble on ribonucleoprotein complexes and regulate virtually all aspects of the gene expression pathway, with profound biological consequences. Third, high-throughput methods of genomic analysis reveal that RNAs other than non-protein-coding RNAs (ncRNAs) represent a major component of the transcriptome that may perform novel functions in gene regulation and beyond. Post-transcriptional regulation, small RNAs and ncRNAs provide an expanding picture of the transcriptome that enriches our views of what genes are, how they operate, evolve and are regulated.
Figures


References
-
- Ast G (2004) How did alternative splicing evolve? Nat Rev Genet 5: 773–782 - PubMed
-
- Bachellerie JP, Cavaille J, Huttenhofer A (2002) The expanding snoRNA world. Biochimie 84: 775–790 - PubMed
-
- Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE (2005) Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell 122: 553–563 - PubMed
-
- Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297 - PubMed
-
- Bartel DP, Chen CZ (2004) Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet 5: 396–400 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

