Drosophila Sex-lethal protein mediates polyadenylation switching in the female germline
- PMID: 16511567
- PMCID: PMC1422161
- DOI: 10.1038/sj.emboj.7601022
Drosophila Sex-lethal protein mediates polyadenylation switching in the female germline
Abstract
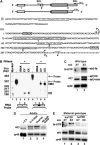
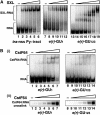
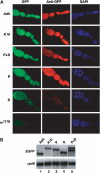
The Drosophila master sex-switch protein Sex-lethal (SXL) regulates the splicing and/or translation of three known targets to mediate somatic sexual differentiation. Genetic studies suggest that additional target(s) of SXL exist, particularly in the female germline. Surprisingly, our detailed molecular characterization of a new potential target of SXL, enhancer of rudimentary (e(r)), reveals that SXL regulates e(r) by a novel mechanism--polyadenylation switching--specifically in the female germline. SXL binds to multiple SXL-binding sites, which include the GU-rich poly(A) enhancer, and competes for the binding of CstF64 in vitro. The SXL-binding sites are able to confer sex-specific poly(A) switching onto an otherwise nonresponsive polyadenylation signal in vivo. The sex-specific poly(A) switching of e(r) provides a means for translational regulation in germ cells. We present a model for the SXL-dependent poly(A) site choice in the female germline.
Figures
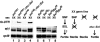


Similar articles
-
Promiscuity in post-transcriptional control of gene expression: Drosophila sex-lethal and its regulatory partnerships.FEBS Lett. 2017 Jun;591(11):1471-1488. doi: 10.1002/1873-3468.12652. Epub 2017 Apr 28. FEBS Lett. 2017. PMID: 28391641 Free PMC article. Review.
-
Induction of female Sex-lethal RNA splicing in male germ cells: implications for Drosophila germline sex determination.Development. 1997 Dec;124(24):5033-48. doi: 10.1242/dev.124.24.5033. Development. 1997. PMID: 9362474
-
Genome-wide identification of alternatively spliced mRNA targets of specific RNA-binding proteins.PLoS One. 2007 Jun 13;2(6):e520. doi: 10.1371/journal.pone.0000520. PLoS One. 2007. PMID: 17565373 Free PMC article.
-
The Drosophila splicing regulator sex-lethal directly inhibits translation of male-specific-lethal 2 mRNA.RNA. 1998 Feb;4(2):142-50. RNA. 1998. PMID: 9570314 Free PMC article.
-
Sex determination in Drosophila: The view from the top.Fly (Austin). 2010 Jan-Mar;4(1):60-70. doi: 10.4161/fly.4.1.11277. Epub 2010 Jan 21. Fly (Austin). 2010. PMID: 20160499 Free PMC article. Review.
Cited by
-
Promiscuity in post-transcriptional control of gene expression: Drosophila sex-lethal and its regulatory partnerships.FEBS Lett. 2017 Jun;591(11):1471-1488. doi: 10.1002/1873-3468.12652. Epub 2017 Apr 28. FEBS Lett. 2017. PMID: 28391641 Free PMC article. Review.
-
Polyadenylation site choice in yeast is affected by competition between Npl3 and polyadenylation factor CFI.RNA. 2007 Oct;13(10):1756-64. doi: 10.1261/rna.607207. Epub 2007 Aug 7. RNA. 2007. PMID: 17684230 Free PMC article.
-
3' end mRNA processing: molecular mechanisms and implications for health and disease.EMBO J. 2008 Feb 6;27(3):482-98. doi: 10.1038/sj.emboj.7601932. EMBO J. 2008. PMID: 18256699 Free PMC article. Review.
-
A-ZIP53, a dominant negative reveals the molecular mechanism of heterodimerization between bZIP53, bZIP10 and bZIP25 involved in Arabidopsis seed maturation.Sci Rep. 2017 Oct 30;7(1):14343. doi: 10.1038/s41598-017-14167-5. Sci Rep. 2017. PMID: 29084982 Free PMC article.
-
The Drosophila Nab2 RNA binding protein inhibits m6A methylation and male-specific splicing of Sex lethal transcript in female neuronal tissue.Elife. 2023 Jul 17;12:e64904. doi: 10.7554/eLife.64904. Elife. 2023. PMID: 37458420 Free PMC article.
References
-
- Amente S, Napolitano G, Licciardo P, Monti M, Pucci P, Lania L, Majello B (2005) Identification of proteins interacting with the RNAPII FCP1 phosphatase: FCP1 forms a complex with arginine methyltransferase PRMT5 and it is a substrate for PRMT5-mediated methylation. FEBS Lett 579: 683–689 - PubMed
-
- Black DL (2003) Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem 72: 291–336 - PubMed
-
- Bopp D, Bell LR, Cline TW, Schedl P (1991) Developmental distribution of female-specific Sex-lethal proteins in Drosophila melanogaster. Genes Dev 5: 403–415 - PubMed
-
- Bopp D, Horabin JI, Lersch RA, Cline TW, Schedl P (1993) Expression of the Sex-lethal gene is controlled at multiple levels during Drosophila oogenesis. Development 118: 797–812 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

