Tbx18 regulates the development of the ureteral mesenchyme
- PMID: 16511601
- PMCID: PMC1386107
- DOI: 10.1172/JCI26027
Tbx18 regulates the development of the ureteral mesenchyme
Abstract
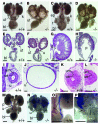
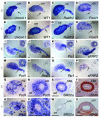
Congenital malformations of the urinary tract are a major cause of renal failure in children and young adults. They are often caused by physical obstruction or by functional impairment of the peristaltic machinery of the ureter. The underlying molecular and cellular defects are, however, poorly understood. Here we present the phenotypic characterization of a new mouse model for congenital ureter malformation that revealed the molecular pathway important for the formation of the functional mesenchymal coating of the ureter. The gene encoding the T-box transcription factor Tbx18 was expressed in undifferentiated mesenchymal cells surrounding the distal ureter stalk. In Tbx18-/- mice, prospective ureteral mesenchymal cells largely dislocalized to the surface of the kidneys. The remaining ureteral mesenchymal cells showed reduced proliferation and failed to differentiate into smooth muscles, but instead became fibrous and ligamentous tissue. Absence of ureteral smooth muscles resulted in a short hydroureter and hydronephrosis at birth. Our analysis also showed that the ureteral mesenchyme derives from a distinct cell population that is separated early in kidney development from that of other mesenchymal cells of the renal system.
Figures





Comment in
-
Going in circles: conserved mechanisms control radial patterning in the urinary and digestive tracts.J Clin Invest. 2006 Mar;116(3):635-7. doi: 10.1172/JCI27985. J Clin Invest. 2006. PMID: 16511599 Free PMC article. Review.
References
-
- Kohaut EC, Tejani A. The 1994 annual report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr. Nephrol. 1996;10:422–434. - PubMed
-
- Woolf AS, Winyard PJ. Molecular mechanisms of human embryogenesis: developmental pathogenesis of renal tract malformations. Pediatr. Dev. Pathol. 2002;5:108–129. - PubMed
-
- Miyazaki Y, Ichikawa I. Ontogeny of congenital anomalies of the kidney and urinary tract, CAKUT. Pediatr. Int. 2003;45:598–604. - PubMed
-
- Chevalier RL. Perinatal obstructive nephropathy. Semin. Perinatol. 2004;28:124–131. - PubMed
-
- Tanagho, E.A. 1981. Development of the ureter. In The ureter. H. Bergman, editor. Springer–Verlag. New York, New York, USA. 1–12.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

